P. de Souto Barreto1,2, J.E. Morley3, W. Chodzko-Zajko4, K.H. Pitkala5, E. Weening-Djiksterhuis6, L. Rodriguez-Mañas7, M. Barbagallo8,9, E. Rosendahl10, A. Sinclair11, F. Landi12, M. Izquierdo13, B. Vellas1,2, Y. Rolland1,2 under the auspices of The International Association of Gerontology and Geriatrics – Global Aging Research Network (IAGG-GARN) and the IAGG European Region Clinical Section
1. Gerontopole of Toulouse, University Hospital of Toulouse (CHU-Toulouse), Toulouse, France; 2. UMR INSERM 1027, University of Toulouse III, Toulouse, France; 3. Divisions of Geriatric Medicine and Endocrinology, Saint Louis University School of Medicine St. Louis, MO, USA; 4. Dean, Graduate College, University of Illinois at Urbana-Champaign, IL, USA; 5. University of Helsinki, Department of General Practice and Helsinki University Hospital, Unit of Primary Health Care; 6. Lectoraat Healthy Aging, Allied Health Care and Nursing, School of Health Care Studies, Hanze University, Groningen, the Netherlands; 7. Service of Geriatrics, Getafe University Hospital, Madrid, Spain; 8. International Association of Gerontology and Geriatrics for the European Region, Chair of the Clinical Section; 9. University of Palermo, Italy. 10; Umeå University, Department of Community Medicine and Rehabilitation, Physiotherapy; 11. University of Aston & Diabetes Frail, UK; 12. Department of Geriatrics, Neurosciences and Orthopedics, Catholic University of the Sacred Heart, Rome, Italy; 13. Public University of Navarre, Department of Health Sciences, Navarra, Pamplona, Spain. Corresponding author: Dr. Philipe de Souto Barreto. Gerontopole of Toulouse, Institute of Ageing. 37, Allées Jules Guesde. 31000 Toulouse, France, Phone number: (+33) 561 145 668, Fax: (+33) 561 145 640 e-mail: philipebarreto81@yahoo.com.br
Abstract
A taskforce, under the auspices of The International Association of Gerontology and Geriatrics – Global Aging Research Network (IAGG-GARN) and the IAGG European Region Clinical Section, composed of experts from the fields of exercise science and geriatrics met in Toulouse, in December 2015, with the aim of establishing recommendations of physical activity and exercise for older adults living in long-term care facilities (LTCF). Due to the high heterogeneity in terms of functional ability and cognitive function that characterizes older adults living in LTCFs, taskforce members established two sets of recommendations: recommendations for reducing sedentary behaviors for all LTCF residents and recommendations for defining specific, evidence-based guidelines for exercise training for subgroups of LTCF residents. In order to promote a successful implementation of recommendations, taskforce experts highlighted the importance of promoting residents’ motivation and pleasure, the key factors that can be increased when taking into account residents’ desires, preferences, beliefs and attitudes toward physical activity and exercise. The importance of organizational factors related to LTCFs and healthcare systems were recognized by the experts. In conclusion, this taskforce report proposes standards for the elaboration of strategies to increase physical activity as well as to prescribe exercise programs for older adults living in LTCFs. This report should be used as a guide for professionals working in LTCFs settings.
Key words: Physical activity, exercise, elderly, long-term care, nursing home, functional ability.
Introduction
Older adults living in long-term care facilities (LTCF) are a complex population, being characterized by high prevalence of dependency in activities of daily living (ADL), multimorbidity, and polymedication (1). Providing the best care for this population represents an immense challenge, particularly in the context of demographic projections for the coming decades in terms of the population ageing. In its recent report (2), the United Nations estimated that the number of people 60 years or over is expected to more than double between 2013 and 2050, with people aged 80 years or over constituting the age-group with the fastest rate of growth. The number of people living in LTCF (3, 4) is also expected to rise, leading to an important increase in health care costs (3-6).
One of the key challenges for the care of the institutionalized elderly is to maintain residents’ functional ability, which is made up of subjects’ intrinsic capacity and environmental characteristics (7), and the ability to cope with their functional limitations for as long as possible. Overall physical activity has been shown to protect against both the incidence of ADL disability and progression of the disability severity in the general population (8). Experts in LTCF research and clinical care, with the support of the International Association of Gerontology and Geriatrics and the World Health Organization, have already recognized the importance of exercise for the quality of care in the LTCF setting (9). Scientific evidence from recent meta-analyses have shown that exercise training, ie, a subset of physical activity that is planned, structured, repetitive, and purposeful, being generally used to improve/maintain physical and functional capacities, has been found to have positive effects on the ability to perform ADLs in LTCF residents (10, 11). Exercise training presents undoubtable advantages since it can positively impact several clinical outcomes that are often present in LTCF residents (eg, falls, cardiovascular diseases, mood disorders), with a low risk of adverse health events. Although exercise undeniably provides health benefits for older people (12), current physical activity guidelines for older adults (see Table 1) were established from a public health perspective (mainly focusing on the primary prevention of non-communicable diseases) (13, 14). Due to its specificities (functional limitations, multimorbidity), the exercise-related objectives for LTCF residents understandably focus more on the maintenance of functional ability and improvements in quality of life than the primary prevention of non-communicable diseases. Therefore, the current physical activity guidelines for older adults are certainly more appropriated for healthy community-dwelling older adults than to very old and vulnerable institutionalized elderly.

Box 1 Summary of key recommendations of physical activity and exercise for older adults in long-term care facilities
*Capable of ambulating/rising from a chair with or without human assistance.
The present article reports on the results of a taskforce held in Toulouse, France, on December 1st 2015, prior to the Nursing Home Research International Working Group Conference (Toulouse, 2 and 3 December, 2015), under the auspices of The International Association of Gerontology and Geriatrics – Global Aging Research Network (IAGG-GARN) and the IAGG European Region Clinical Section. The main objectives of this taskforce, which involved experts from the broad fields of geriatrics, particularly nursing home care, and exercise and sports sciences, particularly physiotherapy and exercise for geriatric populations, were to define strategies to increase physical activity and to establish exercise guidelines for people living in LTCFs. Scientific evidence and feasibility issues for the implementation of physical activity strategies and exercise interventions in a long-term basis in LTCFs were the main pillars for the elaboration of the guidelines reported hereafter.
a. Adapted from: Nelson ME, Rejeski WJ, Blair SN, et al. Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39(8):1435-45; b. Adapetd from: World Health Organization. Global recommendations on physical activity for health. 2010. WHO Press; Geneva, Switzerland.
Healthcare issues in long-term care facilities and potential exercise benefits
Beside ADL dependency, LTCF residents face other important medical challenges. Dementia care, behavioral and psychological symptoms of dementia (BPSD), falls, malnutrition, pain, the use of potentially harmful drugs (eg, antipsychotics), and mood (particularly depression), severe sedentarity (bed- and chair-rest) and quality of life are often recognized by LTCF staff and experts as crucial issues for the care of residents (15, 16).
Exercise training has the potential to improve many of the above mentioned issues.
ADL performance. The most robust information about the positive effects of exercise for people in LTCFs comes from the review and meta-analysis of randomized controlled trials (RCT) from the Cochrane Group (10). In this review, Crocker et al. (10) have found that exercisers had better ability to perform ADL (as measured by the Barthel index or by the Rivermead mobility index) than controls; when pooling the results of all RCTs regardless of the tools used to measure ADL, Crocker et al. (11) found a positive effect of exercise (SMD 0.24, 95% CI 0.11-0.38; p = 0.0005; 13 studies, 2,363 participants), roughly corresponding to an increase of 1.3 points on the Barthel Index 20-point scale; such improvement means, for example, that a person who was dependent on bathing became independent.
Dementia care and cognitive function. Recent systematic reviews suggest that exercise is of benefit for the mobility and physical function of people with dementia (17, 18). Forbes et al. (17), in a meta-analysis of RCTs on exercise for people with dementia, found that exercisers had better ADL than controls (SMD 0.68, 95% CI 0.08 to 1.27, p=0.03; six trials, 289 participants), even though the authors judged this result as of low quality (due to inconsistency and imprecision). Although Forbes et al.’s study was not restricted to LTCF residents, the large majority of the included trials were performed among institutionalized older adults (only 2 out of 17 RCTs were developed among community-dwellers). Mixed results have been found for a potential positive impact of exercise on cognitive function in LTCF residents (10, 17, 18) with no clear and robust conclusions established currently.
Behavioral and Psychological Symptoms of Dementia (BPSD) and depression. Very recent findings from a meta-analysis of RCTs on exercise for treating BPSD in people with dementia did not find a statistically significant effect of exercise on the global levels of BPSD (19). However, the authors found a positive effect of exercise on depressive symptoms, including when restricting analysis to people living in LTCFs (SMD -0.323 (-0.628 to -0.018), p=0.038; six trials, 427 participants); exploratory analysis showed that exercise significantly reduced aberrant motor behavior and provided promising results (not statistically significant) for reducing apathy, agitation, and eating disorders. Although that meta-analysis found a significant effect of exercise on depressive symptoms for people with dementia in LTCFs, the literature on this topic is still mixed. Indeed, while some RCTs in LTCF found that exercise has reduced depressive symptoms (20-22) others found no effect (23-28), and one study showed that exercise increased depressive levels in a subpopulation of people with impaired cognition (29). One of the largest RCTs of exercise designed to reduce depression in residents of care homes (30) found that the intervention (twice/week, 45 minutes, physiotherapist-led group exercise sessions plus a whole home component designed to encourage more physical activity in daily life) was not effective in reducing both depressive symptoms and clinical depression in a 1-year interval; it is important to highlight that participants’ compliance in this study was low (around 50% of exercise sessions, which means that participants really performed exercises in average only once a week).
Falls. Evidence for falls prevention in LTCF through exercise is still mixed. The meta-analysis of RCTs by Cameron and colleagues (31) found no significant effect of exercise (8 RCTs, 1844 participants) on both the rate of falls and the number of fallers. Although the authors suggested that exercise appeared to reduce falls in less frail individuals and to increase falls in frailer people, these results were not significant and were obtained after performing subgroup analysis in a low number of studies (four RCTs for each of “less frail” and “frailer”). Although it is not possible to crudely translate the findings obtained in other populations/settings to the LTCF population, the results of a meta-analysis of RCTs on falls in community-dwelling older adults (32), which included many more RCTs (59 exercise RCTs, 13,264 randomized participants) than the study by Cameron et al. (31), found that multicomponent exercise (a combination of two exercise types or more among: balance/functional training, strength, endurance, flexibility, 3D, general physical activity, or others) reduced both the rate of falls and the risk of falling. Moreover, this study (32) and another meta-analysis of RCTs on fall-induced injuries in older community-dwellers (33), showed that exercise decreased the risk of fractures and injurious falls (including severely injurious falls). Similarly, a review (34)of exercise RCTs in older adults with physical frailty, a very common condition in LTCFs (35, 36) showed that seven out of 10 studies investigating the effects of exercise on incident falls found low rates of falls among exercisers compared to controls.
Quality of life. Evidence for quality of life is very sparse (37) with mixed results across RCTs in the institutionalized elderly. Whereas Lee et al. (38) found improvements in health-related quality of life (both physical and mental components) in a Tai Chi group and Mihalko et al. (39) showed improvements in life satisfaction in a strength exercise group compared to controls, Kerse et al., (29) McMurdo et al. (22) and Conradsson et al. (40) obtained no differences between exercisers and controls after the exercise intervention, although this latter found a positive effect of exercise on subjective well-being in the subgroup of people with dementia at 3-month; Chin A Paw et al. (23) found slight reductions in quality of life among exercisers compared to controls. Although on the basis of the scientific evidence currently available it is impossible to draw any solid conclusions, the positive impact of exercise on key health aspects for older populations, such as improvements on functional ability (10), physical function (including the ability to walk) (10, 41) and depression (19, 42) suggests that exercise should improve quality of life in older people in LTCFs.
Bed- and chair-rest and Sarcopenia. Bed- and chair-rest are important issues in LTCFs, with residents spending around 75% of their waking time in sedentary activities (43, 44). The use of both psychotropic drugs and physical restraints contribute to the high levels of physical inactivity in LTCF residents. Bed- and chair-rest may lead to muscle disuse, which, in turn, increases the risk of sarcopenia (45, 46). Exercise may contribute to decreasing the risk of sarcopenia, which is very prevalent in the institutionalized elderly (47-50). Indeed, since sarcopenia is defined by low muscle mass and low muscle strength/functional impairment (47-51), exercise could decrease sarcopenia in LTCFs by improving muscle strength and overall physical function. Exercise is a well-known powerful intervention for improving strength and physical function in older adults (52) including those living in LTCFs (10). For instance, Crocker et al. (10) showed positive effects of exercise on gait speed in LTCF residents; moreover, most RCTs included in Crocker et al.’s meta-analysis (10) have found positive effects of exercise on strength, particularly in the lower body. Although it is unlikely that exercise alone has positive effects on anorexia, weight loss, or the overall nutritional status of residents, all of them being common clinical conditions in LTCFs that lead to or increase the severity of sarcopenia, some evidence supports the conclusion that exercise plus nutritional supplementation improves the nutritional status of frail older adults (53).
Drugs. Data on the impact of exercise in decreasing the use of harmful drugs, such as antipsychotics, is almost non-existent. In the meta-analysis by de Souto Barreto et al., (19) only four RCTs (three of them in LTCF) out of 20 reported data on changes in antipsychotic use, with no changes in three of them, and mixed results in a fourth RCT; (40) none of the four RCTs reported data on the doses of antipsychotics. In a small RCT, Landi et al. (54) showed that exercise training reduced the use of antipsychotics and hypnotics in cognitively impaired people.
Beside its importance in the treatment/prevention of some of the clinical issues above mentioned, exercise is a recommended intervention for the treatment of several diseases that are prevalent in LTCF residents (55, 56) such as hypertension (57), heart diseases (58, 59) osteoarthritis, (60) osteoporosis (61, 62), diabetes (63), and stroke (64).
In sum, the current evidence clearly shows that exercise delays age-related declines in the ability to perform ADLs and improves physical function in older people living in LTCFs. Although exercise has the potential to improve other health outcomes in this population, such as falls, quality of life, BPSD and depression, the results of RCTs have been mixed and the findings are, therefore, still inconsistent.
Physical activity and exercise recommendations for subgroups of the LTCF population
Exercise may improve the health and daily care of institutionalized older populations. However, older adults living in LTCFs constitute a heterogeneous population. In terms of functional ability, residents may transition from independent living, to assisted living, and finally into skilled nursing care. Similarly for cognitive functioning, residents often transition from intact cognitive function, to mild cognitive impairment, and to dementia with various degrees of severity (from mild to severe dementia). This heterogeneity suggests that a single exercise prescription is unlikely to fit the desires and needs of all LTCF residents.
Taskforce members decided, therefore, to organize their recommendations in two different levels: a first-level of recommendations aiming at reducing sedentary behaviors for all LTCF residents and a second-level set of recommendations that aims to establish relatively specific, evidence-based guidelines for exercise training for well-defined subgroups of LTCF residents.
Improving overall daily life levels of activity among all LTCF residents: first-level of recommendations
Although, to the best of our knowledge, no data is available on the negative role of sedentary behaviors for the health of LTCF residents, limited evidence from community-dwellers suggests that sedentary time (ie, waking time spent in activities that did not increase energy expenditure over 1.5 metabolic equivalents (65), such as watching TV) is associated with adverse health outcomes, including the incidence of both type 2 diabetes and cardiovascular disease, and mortality (66-68). Interestingly, the associations between sedentary time and adverse health events are independent of subjects’ physical activity levels. In a meta-analysis, Chau et al. (68) found a 34% higher mortality risk for adults sitting 10 h/day. Since LTCF residents spent around 75% of their waking time (43, 44) or more than 12hrs/day (69) in sedentary activities, they constitute a target population that might benefit from interventions designed to reduce sedentary time (70).
The increase of daily life activity levels in LTCFs is related to the reduction of sedentary time. In the LTCF setting, it is probably feasible and appropriate to elaborate strategies aiming at reducing sitting time through an increase in light physical activities (71). For example, Slaughter and Estabrooks (72), in a preliminary, quasi-experimental study showed that asking LTCF residents to stand up and sit down as many times as possible once in the morning and once in the evening improved residents’ functional fitness as measured by the 30-seconds chair rise test.
Motivation and pleasure are the key aspects to take into account when attempting to increase overall activity levels in such a population, in whom ADL disability and cognitive impairment are common. To increase residents’ motivation, it is important to build awareness of the importance of replacing sedentary time with physically demanding activities, even if those activities are of light-intensity (eg, walking slowly, light gardening), and LTCF staff should promote residents’ physical engagement during social and daily life activities. Building awareness should target both the older residents themselves as well as LTCF staff, other healthcare professionals (including the primary care physician), residents’ family, and policy makers. To increase motivation and impact overall activity levels the needs of both residents and LTCF staff must be assessed. This assessment of needs will permit LTCF staff to understand potential motivators and barriers to increasing resident activity levels, as well as insight into some of the motivators and barriers related to LTCF staff implementing the appropriate intervention strategies (73, 74).
Proposed recommendations to increase overall activity levels
Although there are no evidence-based guidelines for reducing sedentary time in LTCF settings, taskforce members recognize the crucial importance of enhancing the overall levels of activity in the daily life of residents. LTCF staff leadership should consider:
1) To adopt strategies for breaking the sedentary time of LTCF residents. Establishing short breaks (2-5 minutes) twice or three times a day is probably feasible in the LTCF setting.
2) To systematically use simple strategies to stimulate residents to move: walking to the lunch/dining hall rather than using wheelchairs for people who are able to ambulate, and organizing events that require residents going out from their rooms.
3) To avoid chemical and physical restraints as much as possible since they result in bed and chair-rest.
4) To optimize the utilization of the LTCF architecture and equipment in order to promote mobility.
5) Physical or occupational therapists, or another member of the staff, should organize group activities that are motivating and pleasant. Establishing groups to look after the garden, promoting dancing (75), or organizing walks in green spaces (76) around the LTCF, are examples of potential group activities that can be implemented. Group activities must take into account social affinities among residents, but also residents’ interests and preferences to define the most suitable activities to implement.
6) To use innovative solutions, such as using animal interventions and new technologies, in order to increase residents’ motivation and pleasure and, then, overall activity levels. Animal interventions have been shown to be effective in increasing physical activity in institutionalized older adults (77). The use of robots for the institutionalized elderly has shown to decrease feelings of loneliness (78) and improve participation in activities (79). Robots and other technologies (eg, emotional virtual interfaces) have the potential to increase residents’ motivation for undertaking (light-intensity) physically demanding activities.
Evidence-based exercise guidelines for a subgroup of dependent LTCF residents: second-level of recommendations
Health professionals previously tried to propose some guidance on exercise for LTCF residents (37). However, there is no clear, well-established evidence-based guidelines on exercise for institutionalized older adults currently.
In order to identify the relevant information for debating on the best exercise regimen in LTCF, we decided to focus our critical analysis on ADL disability. Indeed, ADL disability is a major issue for the care of institutionalized older people due to its high prevalence in LTCF (15, 80, 81), its economic burden (3, 4) and its negative repercussion on residents’ quality of life (82). Moreover, the most robust evidence on the health benefits of exercise for older adults in LTCFs regards its positive impact on ADL function (10).
Procedures
We selected relevant exercise RCTs by proceeding as follows: from the most recent review and meta-analysis of RCTs and cluster-RCTs of exercise for people in LTCF by Crocker and colleagues, (10) we identified the RCTs that met two eligibility criteria: 1) had assessed the ability to perform ADLs and 2) used an active exercise intervention; studies of whole-body vibration and staff educational interventions, if not coupled with an exercise training, were not examined. Then, we extracted data from the original RCTs on exercise type, frequency, intensity, session duration, intervention length, and compliance regarding exercise frequency, as well as on the effects of the intervention on ADL performance. To obtain a value that represents the real frequency of exercise practiced by participants, we multiplied compliance rates by exercise frequency and divided it by 100; data on the real frequency are considered when elaborating the exercise recommendations. We additionally examined the articles classified by Crocker et al. (10) as “awaiting assessment” and that were not included in their review. Since Crocker et al (10) have performed their last electronic search on August 2011, we performed an updated search from August 2011 to present (searches were performed on October 13th 2015) using simple key-terms (see Supplementary materials) in PubMed, the Cochrane Central Register of Controlled Trials, SportDiscus, and PsychInfo databases. This electronic search was intended to incorporate recent RCTs that have investigated the effects of exercise on the ability to perform ADL in the LTCF setting; a comprehensive systematic review intending to update Crocker at al.’s study (10) was out of the scope of this taskforce. Although examining all RCTs that met the eligibility criteria, we focused our critical analysis on RCTs that found positive effects of exercise on ADL performance, particularly those with more than 100 participants. The rationale for this procedure is related to the fact that Crocker et al. (11) found that larger RCTs had more conservative results (maybe more realistic results) on the benefits of exercise on the ability to perform ADLs.
After those search procedures, we obtained 32 RCTs that have investigated the effects of exercise on ADL performance in LTCF residents; taskforce members added another exercise RCT meeting the eligibility criteria. Almost all 33 RCTs used measures of functional ability as eligibility criteria for the selection of the study population. Ability to ambulate a few meters (with or without human assistance, according to the study) or ability to rise from a chair (without human assistance, with the assistance of one or even two caregivers, according to the study) were frequent eligibility criteria as well as dependency in basic ADLs (one, two or even more ADLs); therefore, the older LTCF residents participating in those RCTs were dependent in one or several basic ADLs, but were able to ambulate a few meters and/or to rise from a chair. The baseline levels of functional ability of participants in the exercise group in those 33 RCTs can be characterized by an average Barthel index of 68 out of 100 (n=6 studies; (24, 84, 87, 103, 106, 109) varying from 34 to 89.6) or 11.9 out of 20 (n=7 studies; (40, 85, 100-102, 105, 108) varying from 10.1 to 16.1), a functional independence measure (FIM) of 78.1 out of 126 (n=5 studies; (24, 83, 95, 97, 99) varying from 48 to 114.7), or a Rivermead mobility index (RMI) of 5.7 out of 15 (n=4 studies; (85, 100-102) varying from 4.9 to 6.1); for all those measurements, higher scores are better. Regarding cognition, participants of those trials had varied levels of cognitive function, transitioning from intact cognition to severe dementia; at least four studies (84, 86, 87, 96) excluded people with dementia and at least other four studies (28, 103, 107, 109) included only people with dementia. Most studies had participants with some degree of cognitive decline. The baseline mini-mental state examination (MMSE) score for the exercise group was, in average, < 24 in all (24, 25, 27, 28, 40, 83, 85, 92, 95, 98, 102, 103, 107-109) but one study (93) that used this assessment tool; the mean MMSE varied from 6.1 (85) to 25 (93) across studies.
Table 2 shows the characteristics of the 33 studies and their exercise interventions. The most represented countries were the US (n=7), the UK (n=4), and France, Spain and the Netherlands (n=3 each). Mean values for the number of study participants was 135.8, mean aged in average 82.5 years, 74.7% being women. The average exercise program (information for the two exercise groups in Deschamps et al. (92) and Faber et al. (93) and for the three exercise groups in Chin A Paw et al. (23) were combined) lasted around 16.4 weeks, with a weekly frequency of 2.9 sessions (the «daily» frequency of Kerse et al. (29) was replaced by 5), a compliance rate of 83.2% (n=22 RCTs), and a session duration of 42 minutes; real frequency performed (frequency X compliance/100) was 2.3 times/week. The main type of exercise was by far a moderate-intensity multicomponent training (a combination of two or more exercise types, such as balance, strength, aerobic, flexibility); the exercise types used across studies were strength (n=25), balance/coordination (n=17), aerobic/cardiorespiratory endurance (n=16), and flexibility (n=15). None of the trials reported major adverse events (death, important cardiovascular events (eg, heart attack), severely injurious falls, or fractures) related to the exercise intervention; at least two studies (27, 28) reported that exercisers fell more than controls, but this difference did not reach statistical significance in any of the trials. It must be, however, highlighted that not all studies clearly reported adverse events.
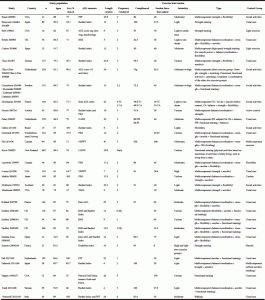
Table 2 Study characteristics and exercise interventions. Studies showing a positive effect of exercise, compared to controls, on ADL performance (global scores or scores of validated subscales) are italicized
Note. ADL, activities of daily living; CA, cognition-action group; FIM, functional independence measure; FW, functional walking group; GARS, Groningen Activity Restriction Scale; IB, in balance group; LLFDI, Late Life Function and Disability Instrument; MDS, minimum data set; NHPPT, Nursing Home Physical Performance Test; PADL, Physical Activities of Daily Living; PPT, physical performance test; RMI, Rivermead Mobility Index; TC, Tai Chi; a. When participants’ age was provided separately by group, we added all ages and divided by the number of study groups to obtain a mean age; b If % of female provided separately by group, we added all % of female and divided by the number of study groups to obtain a mean. Sex may also have been calculated from the absolute number of males and females in the studies; c. If intervention length was provided in months, we then multiplied the months by 4.3 to obtain «weeks»; d. If this information was provided as a range, we calculated a mean by adding upper and lower limits and dividing by two; e. In those studies, intensity was not clearly described by the authors. When possible, we defined the intensity from “cues” provided by the authors. For example, in the study by Baum 2003 rated as moderate: «Each exercise was begun with one set of 5 repetitions and gradually progressed to two sets of 10 as tolerated. Participants were evaluated weekly for the quality of their movement and number of repetitions with good technique to determine progression of soft weights, balls, or resistance of the therabands.»; f. Before the crossing over of the interventions; g. Information on the 3 exercise groups (strength training, functional training, and a combination of strength and functional training) were combined because none of these groups improved ADL function compared to controls. This allowed us to obtain a mean value for the three groups; h. Information regards only the Wii part of the intervention; i. Although the «mean mobility scores (as measured by the Rivermead Mobility Index) declined in the control group (n:16; baseline: 5.9, six weeks: 4.75) when compared to the intervention group (n:17;baseline: 6.1, six weeks: 6.2)», the authors did not present any formal statistical analysis; j. The exact weekly frequency was not provided, but the authors reported the total exposure (number of visits and total duration). From this, we calculated average weekly frequency and session duration
Fifteen RCTs found a positive effect of exercise on measures of ADL performance (studies italicized in Table 2). The characteristics of these RCTs were similar to those described for the whole sample: the average number of study participants was 124.5, aged 82.8 years, 73.4% women. The average exercise program lasted almost 24 weeks, with a weekly frequency of 3.1 sessions, a compliance rate of 81.7% (n=12 RCTs), and a session duration of about 48 minutes; real frequency performed was 2.5 times/week. The main type of exercise was by far a moderate-intensity multicomponent training; the exercise types more used across studies were strength (n=12), aerobic/cardiorespiratory endurance (n=8), flexibility (n=9), and balance/coordination (n=6). Focusing on the seven RCTs in which the exercise intervention was found to improve ADL function and that had more than 100 participants, we obtained the following results: the mean number of study participants was 209, aged around 82 years, 72.9% women. The average exercise program lasted almost 27 weeks, with a weekly frequency of 3.1, a compliance rate of 68% (n=5 RCTs), and a session duration of 34 minutes; real frequency performed was 2.3 times/week. The main type of exercise was a moderate-intensity multicomponent training (all seven studies used a multicomponent training); the exercise types more used across studies were strength (n=6), aerobic/cardiorespiratory endurance (n=6), flexibility (n=4), and balance/coordination (n=3).
Proposed guidelines for exercise training
The evidence shows that a moderate-intensity multicomponent training program, composed of strength and aerobic exercises, is effective for improving ADL performance in older adults living in LTCFs; additional benefits may be achieved by integrating balance/coordination and flexibility exercises to the multicomponent training. The guidelines proposed below should be applied for institutionalized older adults who are dependent in basic ADLs, but still able to ambulate and/or to rise from a chair (with or without human assistance), including people who had cognitive decline and even dementia; indeed, the evidence used to build these guidelines was extracted from populations with such characteristics, as described above. Moreover, these recommendations should be applied for overall stable patients. Patients in the end-of-life, bed-bound, or with any unstable conditions precluding exercise should be examined on a case-by-case basis and should have a more personalized intervention, if appropriate. In order to be effective in a long-term basis, the exercise program must be continuously adapted to individuals’ capacity and must be organized as a progressive challenge. Residents should be stimulated to comply with these guidelines and clear strategies to «re-engage» those who quit exercise classes should be defined by the LTCF staff leadership.
The taskforce group makes the following recommendations as the minimum exercise training to be provided for this subgroup of LTCF residents:
Exercise type. Multicomponent training. Exercises designed for improving muscle strength and cardiorespiratory endurance must be the essential part of the multicomponent training. Strength training may be performed through weight-bearing exercises and using free weights (the use of machines is less usual in the LTCF setting, even though it is feasible). . Special attention must be done to strengthening the lower-body. The aerobic aspect may traditionally be implemented through walking (in continuum or by walk bouts); using circuit training, with walk bouts interposed between other types of exercises, may also contribute to achieve aerobic endurance. Other exercise types, particularly flexibility and balance, should be added to the exercise program whenever possible. For example, active range-of-motion exercises may be integrated in the warm-up phase, while stretching may be part of the cool down phase of the training. In order to increase strength and, then, self-confidence and mastery, several sessions of strength training may be needed before introducing balance/coordination activities, particularly for very vulnerable people. Specific exercises may be integrated in order to train people for the execution of actions that are strongly associated with falls, particularly standing quietly, sitting down or lowering, and initiation of walking (110); adding a component of muscle power (prioritizing the speed of muscle contraction) may be integrated in such an exercise training. Walking forward with direction changes may also contribute to reduce falls, since walking forward is the most common activity at the moment of fall (110).
Intensity. Moderate. Moderate-intensity can be achieved in the following ways for the different types of exercise:
Strength. One or two sets of exercises, performed at 13-15 repetitions maximum. The number of exercises will be limited by time availability and residents’ exercise capacity; the ideal number of exercises would be of 8-10. Low-intensity exercises performed at up to 20 repetitions maximum may be required, particularly in the first weeks of the exercise program. High-intensity exercise, ie, exercises performed at 8-12 repetitions maximum, can be executed, but it may require a closer monitoring (90).
Aerobic endurance. Through exercises that noticeably increase heart rate and respiration, without generating breathlessness or undue fatigue. It can be easily monitored through self-report as recommended by ACSM/AHA: «On a 10-point scale, where sitting is 0 and all-out effort is 10, moderate-intensity activity is a 5 or 6 and produces noticeable increases in heart rate and breathing» (13). Attention must be paid for people with important cognitive impairment, who are unable to identify moderate intensity and to communicate potential acute signs, such as pain. Light-intensity exercises appear to provide some benefits and should be envisaged for very vulnerable people and at the start of any exercise program (during the first weeks of exercise). High-intensity exercise can be performed, but it may require a closer monitoring; on the same self-reported scale as above, «vigorous-intensity activity is a 7 or 8 and produces large increases in heart rate and breathing» (13).
Flexibility. Active range-of-motion and stretch exercises should be performed during 10-30 seconds per exercise; the number of exercises will be limited by time constraints. Although assessing the intensity of flexibility exercises is very difficult, asking residents to perform range-of-motion and stretching exercises to the maximum of their capacity without reaching painful levels is probably feasible and safe. Attention must be paid to people who are unable to communicate painful feelings.
Balance. Establishing the intensity of balance exercises is a challenge since there are no validated instruments developed to this purpose (111). A progressive increase in the difficulty for executing static (eg, semi-tandem, tandem, single-leg stand), but mainly dynamic balance (eg, walking in the line, tandem walking in the line; walking with changing directions slowly and, then, faster) is required. Exercises that reduce sensory input (e.g., standing with eyes closed) (12) can also be used.
Frequency. Twice a week. Doing more than twice a week can be a difficult target for most LTCF residents, and doing less than that can be ineffective (88). An interval of at least 48hrs between sessions should be respected. An exercise program with three or more weekly sessions is, however, safe and may be feasible for fitter residents.
Duration. Between 35 and 45 minutes per session. This session duration range gives enough time for the essential components of the exercise training, ie, strength and aerobic training, and still leaves some time for other exercises, such as flexibility and balance. Lesser durations may be needed for very vulnerable people and during the first weeks of exercise. Longer sessions (preferentially, not longer than 60 minutes) are, however, feasible for most people.
A typical exercise session for older people in LTCFs
We provide below an example of how a 45-minute session of exercise could be divided:
• 4 minutes warm-up. Range-of-motion exercises (for example, for the wrists, shoulders, hip, knees, and ankles), followed by light walking;
• 8 minutes balance/coordination. Standing balance with increasing difficulty (eg, narrowing the base of support); activities of bodyweight shift; walking forward with changing directions; walking along a straight line (forward, backward, and sideward).
• 15 minutes strength. 13-15 repetitions maximum of chair rises, with increasing difficulty (eg, emphasizing speed of movement); different theraband exercises for the upper-body and trunk; calf weights for knee extension and flexion or weighted belts for functional lower-limb strength exercises.
• 15 minutes aerobic. Five 3-minute bouts of walking interposed between two strength exercises and/or between two balance/coordination exercises.
• 3 minutes calm down. Very light walking followed by a few stretching exercises.
Implementing the recommendations
To promote the implementation of the proposed recommendations in the real life of LTCFs, it is crucial to take into account residents’ desires, preferences, beliefs and attitudes toward physical activities and exercises. For instance, taking into account subject’s self-efficacy (ie, one’s belief that he/she is capable of performing the goal-directed behaviour) to define challenging but feasible goals (with the resident whenever possible) are essential factors to consider when designing strategies to increase/maintain optimal levels of physical activity and exercise. Other important aspects for the successful implementation of the recommendations are related to promoting social support (eg, by doing physical activities and exercise in group), and providing a stimulating environment (eg, by using different equipment with different colors: free weights, balls, carpets with different consistencies, by using music during the exercise sessions, and even implementing dual-task training) for LTCF residents. Building awareness of residents’ family members and primary healthcare providers about the importance of physical activity and exercise for older adults as well as about their role in keeping the resident physically active is a key aspect for the implementation of our recommendations.
Moreover, LTCF organizational aspects are as important as the recommendations themselves. Even though it is out of the scope of this taskforce to deeply debate about LTCF organizational aspects, taskforce members recognize that strategies that may favor a successful exercise implementation in LTCFs should be discussed in the facility and healthcare system levels. In the facility level, LTCF leadership staff may discuss and implement different strategies that include (but are not limited to): involving residents’ family and primary health care provider in the “exercise strategy” to increase residents’ adherence to exercise; adding exercise programs in the personalized healthcare plan of all residents who do not have any contraindication for exercising (every resident who has no contraindications must have a personalized exercise program); defining strategies for providing incentives to residents who exercise regularly, for example, by rewarding residents who increased or maintained high attendance rates in exercise sessions; defining an “exercise referent” person among LTCF staff, as it already exists for pain care; providing sufficient training to LTCF staff and volunteers in how to conduct exercise sessions. To optimize the potential health benefits of physical activity and exercise in LTCF residents, extra people in the LTCF is required. The training level in terms of knowledge and experience in physical activity of the extra people can vary from highly trained professionals to sufficiently trained volunteers. Although the ideal picture is that exercise training sessions in the LTCF should be led by professionals from the field of exercise sciences, and all efforts must be undertaken towards this ideal picture, an important number of facilities (maybe most of them) will not have the required resources for that; in those LTCFs, healthcare providers, other than exercise science professionals, and/or volunteers will be in charge of conducting exercise sessions, needing thus to achieve minimum levels of knowledge and expertise on exercise.
In a transition level, which would ask for efforts from both facility and healthcare systems, strategies that build awareness about the potential role of LTCF as a site for healthcare prevention through exercise should be promoted; for example, by taking advantage of the already existing space and staff expertise in LTCFs, facilities may open its doors and invite the whole community to exercise in the LTCF, with LTCF residents. Other innovative solutions, such as integrating volunteers, including high-school or college students, into the strategies of the LTCF in order to increase both residents’ daily life physical activity (eg. walking or gardening with volunteers) and their adherence to exercise sessions (eg, exercising with volunteers) should also be welcome; volunteer-led exercise interventions have already been tested and provided positive results on exercise adherence (participants completed in average 94% of the proposed volunteer-led sessions) and on clinical outcomes (eg, ADL performance) (112).
In the level of healthcare systems, policies that facilitate the implementation of exercise in the LTCF setting should be debated and implemented. For example, establishing the successful organization of exercise sessions in the LTCF (for example, by conducting exercises according to the guidelines proposed herein) as a quality indicator of care and/or creating a distinguished label (“LTCF fit-friendly”) for LTCFs that organize exercise successfully, and defining ways to pay/reimburse the expenses related to the exercise program, would certainly help promoting exercise in LTCF.
Conclusions and Perspectives
Scientific evidence about the effects of physical activity and exercise on the health of older adults in LTCF has increased in the past decade. Establishing strategies to increase activity levels in daily life as well as minimal standards in terms of exercise regimen is important for the guidance of professionals working in this setting.
Taskforce members purposefully covered a very large spectrum of recommendations from overall strategies to increase physical activity levels in the daily life of LTCF residents to more precise guidelines on exercise training to a well-defined subgroup of LTCF residents. Our recommendations should not be used as a one-size-fits-all approach. Instead, they should be used as a flexible framework since the most appropriate strategies to increase residents’ daily life physical activity will vary across facilities as well as the different parts of the exercise regimen (type, frequency, intensity, duration) should be adapted according to residents’ capacities, needs and desires. It is important to highlight that, currently, LTCF residents appear to engage in less exercise than the recommendations proposed herein, as showed in an observational cross-sectional study in which only 10% of nursing home residents exercised at least twice a week (113).
In order to confirm that our recommendations in terms of overall physical activity and exercise training are appropriate and effective for institutionalized older adults, further studies are needed. Well-designed and large RCTs are particularly welcome to examine the effectiveness of the exercise recommendations on different relevant outcomes for LTCF residents, such as BPSD, falls, malnutrition, and quality of life, as well as on staff-related outcomes, such as reductions of staff burnout. Cost-effectiveness analysis is also required in order to strengthen our recommendations.
Conflicts of Interest: All authors declare no conflicts of interest.
Please, note that this article will be jointly published with the Journal of the American Medical Directors Association (JAMDA)
References
1. De Souto Barreto P, Lapeyre-Mestre M, Mathieu C, et al. A multicentric individually-tailored controlled trial of education and professional support to nursing home staff: research protocol and baseline data of the IQUARE study. J Nutrit Health Aging. 2013; 17(2): 173-178.
2. United Nations, Department of Economic and Social Affairs, Population Division. World Population Ageing 2013. 2013;ST/ESA/SER.A/348.
3. Pickard L, Comas-Herrera A, Costa-Font J, et al. Modelling an entitlement to longterm care services for older people in Europe: projections for long-term care expenditure to 2050. J Eur Soc Policy. 2007; 17:33-48.
4. De Meijer CAM, Majer IM, Koopmanschap MA, Van Baal PH. Forecasting lifetime and aggregate long-term care spending: accounting for changing disability patterns. Med Care. 2012;50(8):722‑729.
5. Lubitz J, Cai L, Kramarow E, Lentzner H. Health, life expectancy, and health care spending among the elderly. N Engl J Med. 2003;349:1048–55.
6. Wübker A, Zwakhalen SM, Challis D, et al. Costs of care for people with dementia just before and after nursing home placement: primary data from eight European countries. Eur J Health Econ. 2015;16(7):689-707.
7. World Health Organization. World report on ageing and health. WHO Press: Geneva; 2015. pp. 247
8. Tak E, Kuiper R, Chorus A, Hopman-Rock M. Prevention of onset and progression of basic ADL disability by physical activity in community dwelling older adults: a meta-analysis. Ageing Res Rev. 2013;12(1):329-38.
9. Tolson D, Rolland Y, Andrieu S, et al. International Association of Gerontology and Geriatrics: a global agenda for clinical research and quality of care in nursing homes. J Am Med Dir Assoc. 2011;12(3):184-9.
10. Crocker T, Forster A, Young J, et al. Physical rehabilitation for older people in long-term care. Cochrane Database Syst Rev. 2013;2:CD004294.
11. Crocker T, Young J, Forster A, et al. The effect of physical rehabilitation on activities of daily living in older residents of long-term care facilities: systematic review with meta-analysis. Age Ageing. 2013;42(6):682-8.
12. American College of Sports Medicine, Chodzko-Zajko WJ, Proctor DN, Fiatarone Singh MA, et al. American College of Sports Medicine position stand. Exercise and physical activity for older adults. Med Sci Sports Exerc. 2009;41(7):1510-30.
13. Nelson ME, Rejeski WJ, Blair SN, et al. Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39(8):1435-45.
14. World Health Organization. Global recommendations on physical activity for health. 2010. WHO Press; Geneva, Switzerland: p. 58.
15. Rolland Y, Abellan van Kan G, Hermabessiere S, et al. Descriptive study of nursing home residents from the REHPA network. J Nutr Health Aging. 2009;13(8):679-83.
16. Morley JE, Caplan G, Cesari M, et al. International survey of nursing home research priorities. J Am Med Dir Assoc. 2014;15(5):309-12.
17. Forbes D, Forbes SC, Blake CM, et al. Exercise programs for people with dementia. Cochrane Database Syst Rev. 2015;4:CD006489.
18. Pitkälä K, Savikko N, Poysti M, Strandberg T, Laakkonen M-L. Efficacy of physical exercise intervention on mobility and physical functioning in older people with dementia: a systematic review. Exp Gerontol. 2013;48(1):85–93
19. de Souto Barreto P, Demougeot L, Pillard F, et al.Exercise training for managing behavioral and psychological symptoms in people with dementia: A systematic review and meta-analysis. Ageing Res Rev. 2015. 24:274-285.
20. Buettner LL, Ferrario J. Therapeutic recreation-nursing team: a therapeutic intervention for nursing home residents with dementia. Annual in Therapeutic Recreation. 1997–1998;7:21–8.
21. Brill P, Jensen R, Koltyn K, et al. The feasibility of conducting a group-based progressive strength training program in residents of a multi-level care facility. Act Adapt Aging. 1998; 22(4):53–63.
22. McMurdo MET, Rennie LM. A controlled trial of exercise by residents of old people’s homes. Age Ageing. 1993;22:11–5.
23. Chin A Paw MJ, van Poppel MNM, Twisk JWR, van Mechelen W. Effects of resistance and all-round, functional training on quality of life, vitality and depression of older adults living in long-term care facilities: a ’randomized’ controlled trial. BMC Geriatrics. 2004;4:5.
24. Dorner T, Kranz A, Zettl-Wiedner K, et al. The effect of structured strength and balance training on cognitive function in frail, cognitive impaired elderly long-term care residents. Aging Clin Exp Res. 2007;19(5):400–5.
25. Meuleman JR, Brechue WF, Kubilis PS, Lowenthal DT. Exercise training in the debilitated aged: strength and functional outcomes. Arch Phys Med Rehabil. 2000;81:312–8.
26. Morris JN, Fiatarone M, Kiely DK, et al. Nursing rehabilitation and exercise strategies in the nursing home. J Gerontol A Biol Sci Med Sci. 1999;54(10):M494-500.
27. Mulrow CD, Gerety MB, Kanten D, et al. A randomized trial of physical rehabilitation for very frail nursing home residents. JAMA. 1994;271(7):519–24.
28. Rolland Y, Pillard F, Klapouszczak A, et al. Exercise program for nursing home residents with Alzheimer’s disease: a 1-year randomized, controlled trial. J Am Geriatr Soc 2007; 55: 158–65.
29. Kerse N, Peri K, Robinson E, et al. Does a functional activity programme improve function, quality of life, and falls for residents in long term care? Cluster randomised controlled trial. BMJ. 2008 9;337:a1445.
30. Underwood M, Lamb SE, Eldridge S, et al. Exercise for depression in elderly residents of care homes: a cluster-randomised controlled trial. Lancet. 2013;382(9886):41-9.
31. Cameron ID, Gillespie LD, Robertson MC, et al. Interventions for preventing falls in older people in care facilities and hospitals. Cochrane Database Syst Rev. 2012 Dec 12;12:CD005465.
32. Gillespie LD, Robertson MC, Gillespie WJ, et al. Interventions for preventing falls in older people living in the Community. Cochrane Database Syst Rev. 2012 Sep 12;9:CD007146.
33. El-Khoury F, Cassou B, Charles MA, Dargent-Molina P. The effect of fall prevention exercise programmes on fall induced injuries in community dwelling older adults: systematic review and meta-analysis of randomised controlled trials. BMJ. 2013;347:f6234.
34. Cadore EL, Rodríguez-Mañas L, Sinclair A, Izquierdo M. Effects of different exercise interventions on risk of falls, gait ability, and balance in physically frail older adults: a systematic review. Rejuvenation Res. 2013;16(2):105–14
35. Kojima G. Prevalence of Frailty in Nursing Homes: A Systematic Review and Meta-Analysis. J Am Med Dir Assoc. 2015 1;16(11):940–5.
36. Luo H, Lum TYS, Wong GHY, Kwan JSK, Tang JYM, Chi I. Predicting Adverse Health Outcomes in Nursing Homes: A 9-Year Longitudinal Study and Development of the FRAIL-Minimum Data Set (MDS) Quick Screening Tool. J Am Med Dir Assoc. 2015 In Press.
37. Weening-Dijksterhuis E, de Greef MH, Scherder EJ, et al. Frail Institutionalized Older Persons: A Comprehensive Review on Physical Exercise, Physical Fitness, Activities of Daily Living, and Quality-of-Life. Am J Phys Med Rehabil. 2011;90(2):156-68.
38. Lee LY, Lee DT, Woo J. Tai Chi and Health-Related Quality of Life in Nursing Home Residents. J Nurs Scholarsh. 2009;41(1):35-43.
39. Mihalko SL, McAuley E. Strength training effects on subjective well-being and physical function in the elderly. J Aging Phys Act. 1996;4:56–68.
40. Conradsson M, Littbrand H, Lindelof N, et al. Effects of a high-intensity functional exercise programme on depressive symptoms and psychological well-being among older people living in residential care facilities: A cluster-randomized controlled trial. Aging Ment Health 2010; 14: 565–76.
41. Giné-Garriga M, Roqué-Fíguls M, Coll-Planas L, et al. Physical Exercise Interventions for Improving Performance-Based Measures of Physical Function in Community-Dwelling, Frail Older Adults: A Systematic Review and Meta-Analysis. Arch Phys Med Rehabil. 2014;95(4):753-769.e3.
42. Blake H, Mo P, Malik S, Thomas S. How effective are physical activity interventions for alleviating depressive symptoms in older people? A systematic review. Clin Rehabil. 2009;23(10):873-87.
43. Chin A Paw MJM, van Poppel MNM, van Mechelen W. Effects of resistance and functional-skills training on habitual activity and constipation among older adults living in long-term care facilities: a randomized controlled trial. BMC Geriatr. 2006;6:9.
44. Ikezoe T, Asakawa Y, Shima H, Kishibuchi K, Ichihashi N. Daytime physical activity patterns and physical fitness in institutionalized elderly women: an exploratory study. Arch Gerontol Geriatr. 2013;57(2):221‑5.
45. Tanner RE, Brunker LB, Agergaard J, Barrows KM, Briggs RA, Kwon OS, et al. Age-related differences in lean mass, protein synthesis and skeletal muscle markers of proteolysis after bed rest and exercise rehabilitation. J Physiol. 2015;593(18):4259–73
46. Wall BT, Dirks ML, van Loon LJC. Skeletal muscle atrophy during short-term disuse: implications for age-related sarcopenia. Ageing Res Rev. 2013;12(4):898–906
47. Senior HE, Henwood TR, Beller EM, et al. Prevalence and risk factors of sarcopenia among adults living innursing homes. Maturitas. 2015;82(4):418-23.
48. Smoliner C, Sieber CC, Wirth R. Prevalence of Sarcopenia in Geriatric Hospitalized Patients. J Am Med Dir Assoc. 2014;15(4):267-72.
49. Yalcin A, Aras S, Atmis V, et al. Sarcopenia prevalence and factors associated with sarcopenia in older people living in a nursing home in Ankara Turkey. Geriatr Gerontol Int. 2015 ; In Press. doi: 10.1111/ggi.12570.
50. Landi F, Liperoti R, Fusco D, et al. Sarcopenia and mortality among older nursing home residents. J Am Med Dir Assoc. 2012;13(2):121-6.
51. Cesari M, Fielding R, Bénichou O, et al. Pharmacological interventions in frailty and sarcopenia: report by the international conference on frailty and sarcopenia research task force. J Frailty Aging. 2015;4(3):114-120.
52. Liu CJ, Latham NK. Progressive resistance strength training for improving physical function in older adults. Cochrane Database Syst Rev. 2009;(3):CD002759.
53. Tieland M, Dirks ML, van der Zwaluw N, et al. Protein Supplementation Increases Muscle Mass Gain During Prolonged Resistance-Type Exercise Training in Frail Elderly People: A Randomized, Double-Blind, Placebo-Controlled Trial. J Am Med Dir Assoc. 2012;13(8):713-9.
54. Landi F, Russo A, Bernabei R. Physical activity and behavior in the elderly: a pilot study. Arch Gerontol Geriatr Suppl 2004; 235–41.
55. U.S. Department of Health and Human Services Centers for Disease Control and Prevention. Quick Stats: Ten Most Common Chronic Conditions Among Persons Living in Residential Care Facilities — National Survey of Residential Care Facilities, United States, 2010. MMWR Morb Mortal Wkly Rep. 2012; 61:603.
56. Moore KL, Boscardin WJ, Steinman MA, Schwartz JB. Patterns of chronic co-morbid medical conditions in older residents of U.S. nursing homes: differences between the sexes and across the agespan. J Nutr Health Aging. 2014;18(4):429-36.
57. Pescatello LS, MacDonald HV, Lamberti L, Johnson BT. Exercise for Hypertension: A Prescription Update Integrating Existing Recommendations with Emerging Research. Curr Hypertens Rep. 2015;17(11):87.
58. American College of Sports Medicine position stand. Exercise for patients with coronary artery disease. Med Sci Sports Exerc. mars 1994;26(3):i ‑ v.
59. Gielen S, Laughlin MH, O’Conner C, Duncker DJ. Exercise training in patients with heart disease: review of beneficial effects and clinical recommendations. Prog Cardiovasc Dis. 2015;57(4):347‑55.
60. Focht BC. Effectiveness of Exercise Interventions in Reducing Pain Symptoms Among Older Adults With Knee Osteoarthritis: A Review. J Aging Phys Act. 2006;14(2):212-35.
61. Giangregorio LM, Papaioannou A, Macintyre NJ, Ashe MC, Heinonen A, Shipp K, et al. Too Fit To Fracture: exercise recommendations for individuals with osteoporosis or osteoporotic vertebral fracture. Osteoporos Int. 2014;25(3):821‑35.
62. Giangregorio LM, McGill S, Wark JD, Laprade J, Heinonen A, Ashe MC, et al. Too Fit To Fracture: outcomes of a Delphi consensus process on physical activity and exercise recommendations for adults with osteoporosis with or without vertebral fractures. Osteoporos Int. 2015;26(3):891‑910
63. Colberg SR, Albright AL, Blissmer BJ, Braun B, Chasan-Taber L, Fernhall B, et al. Exercise and type 2 diabetes: American College of Sports Medicine and the American Diabetes Association: joint position statement. Exercise and type 2 diabetes. Med Sci Sports Exerc. déc 2010;42(12):2282‑303.
64. Billinger SA, Arena R, Bernhardt J, Eng JJ, Franklin BA, Johnson CM, et al. Physical activity and exercise recommendations for stroke survivors: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. août 2014;45(8):2532‑53.
65. Mansoubi M, Pearson N, Clemes SA, Biddle SJ, Bodicoat DH, Tolfrey K, et al. Energy expenditure during common sitting and standing tasks: examining the 1.5 MET definition of sedentary behaviour. BMC Public Health. 2015;15:516
66. van der Ploeg HP, Chey T, Korda RJ, Banks E, Bauman A. Sitting time and all-cause mortality risk in 222 497 Australian adults. Arch Intern Med. 2012;172(6):494‑500.
67. Grøntved A, Hu FB. Television viewing and risk of type 2 diabetes, cardiovascular disease, and all-cause mortality: a meta-analysis. JAMA. 2011;305(23):2448‑55.
68. Chau JY, Grunseit AC, Chey T, Stamatakis E, Brown WJ, Matthews CE, et al. Daily sitting time and all-cause mortality: a meta-analysis. PLoS ONE. 2013;8(11):e80000.
69. Keogh JW, Senior H, Beller EM, Henwood T. Prevalence and Risk Factors for Low Habitual Walking Speed in Nursing Home Residents: An Observational Study. Arch Phys Med Rehabil. 2015;96(11):1993‑9.
70. De Souto Barreto P. Non-pharmacological interventions in the nursing home setting: doest it make any sense to struggle against sedentary behavior among residents? J Nursing Home Res. 2015;1:52-54.
71. Sparling PB, Howard BJ, Dunstan DW, Owen N. Recommendations for physical activity in older adults. BMJ. 2015;350:h100.
72. Slaughter SE, Estabrooks CA. Optimizing the mobility of residents with dementia: a pilot study promoting healthcare aide uptake of a simple mobility innovation in diverse nursing home settings. BMC Geriatr. 2013;13:110.
73. Baert V, Gorus E, Calleeuw K, De Backer W, Bautmans I. An Administrator’s Perspective on the Organization of Physical Activity for Older Adults in Long-Term Care Facilities. J Am Med Dir Assoc. 2016 Jan 1;17(1):75–84.
74. Baert V, Gorus E, Guldemont N, De Coster S, Bautmans I. Physiotherapists’ perceived motivators and barriers for organizing physical activity for older long-term care facility residents. J Am Med Dir Assoc. 2015 May 1;16(5):371–9.
75. Vankova H, Holmerova I, Machacova K, Volicer L, Veleta P, Celko AM. The effect of dance on depressive symptoms in nursing home residents. J Am Med Dir Assoc. 2014 Aug;15(8):582–7.
76. Moran M, Van Cauwenberg J, Hercky-Linnewiel R, Cerin E, Deforche B, Plaut P. Understanding the relationships between the physical environment and physical activity in older adults: a systematic review of qualitative studies. Int J Behav Nutr Phys Act. 2014;11:79.
77. Friedmann E, Galik E, Thomas SA, Hall PS, Chung SY, McCune S. Evaluation of a pet-assisted living intervention for improving functional status in assisted living residents with mild to moderate cognitive impairment: a pilot study. Am J Alzheimers Dis Other Demen. 2015 May;30(3):276–89.
78. Robinson H, Macdonald B, Kerse N, Broadbent E. The psychosocial effects of a companion robot: a randomized controlled trial. J Am Med Dir Assoc. 2013 Sep;14(9):661–7
79. Sung H-C, Chang S-M, Chin M-Y, Lee W-L. Robot-assisted therapy for improving social interactions and activity participation among institutionalized older adults: a pilot study. Asia-Pac Psychiatry Off J Pac Rim Coll Psychiatr. 2015 Mar;7(1):1–6.
80. de Souto Barreto P, Lapeyre-Mestre M, Mathieu C, Piau C, Bouget C, Cayla F, et al. A multicentric individually-tailored controlled trial of education and professional support to nursing home staff: research protocol and baseline data of the IQUARE study. J Nutr Health Aging. 2013;17(2):173‑8.
81. Rolland Y, Andrieu S, Crochard A, Goni S, Hein C, Vellas B. Psychotropic drug consumption at admission and discharge of nursing home residents. J Am Med Dir Assoc. 2012;13(4):407.e7‑12.
82. Andersen CK, Wittrup-Jensen KU, Lolk A, Andersen K, Kragh-Sørensen P. Ability to perform activities of daily living is the main factor affecting quality of life in patients with dementia. Health Qual Life Outcomes. 2004;2:52.
83. Baum EE, Jarjoura D, Polen AE, Faur D, Rutecki G. Effectiveness of a group exercise program in a long-term care facility: a randomized pilot trial. J Am Med Dir Assoc. avr 2003;4(2):74‑80.
84. Benavent-Caballer V, Rosado-Calatayud P, Segura-Ortí E, Amer-Cuenca JJ, Lisón JF. Effects of three different low-intensity exercise interventions on physical performance, muscle CSA and activities of daily living: a randomized controlled trial. Exp Gerontol. oct 2014;58:159‑65.
85. Brittle N, Patel S, Wright C, Baral S, Versfeld P, Sackley C. An exploratory cluster randomized controlled trial of group exercise on mobility and depression in care home residents. Clin Rehabil. 2009;23(2):146‑54.
86. Cadore EL, Casas-Herrero A, Zambom-Ferraresi F, Idoate F, Millor N, Gómez M, et al. Multicomponent exercises including muscle power training enhance muscle mass, power output, and functional outcomes in institutionalized frail nonagenarians. Age Dordr Neth. 2014;36(2):773–85
87. Chen K-M, Li C-H, Chang Y-H, Huang H-T, Cheng Y-Y. An elastic band exercise program for older adults using wheelchairs in Taiwan nursing homes: a cluster randomized trial. Int J Nurs Stud. 2015;52(1):30‑8.
88. Chin A Paw MJM, van Poppel MNM, Twisk JWR, van Mechelen W. Once a week not enough, twice a week not feasible? A randomised controlled exercise trial in long-term care facilities [ISRCTN87177281]. Patient Educ Couns. oct 2006;63(1-2):205‑14.
89. Rosendahl E, Lindelöf N, Littbrand H, Yifter-Lindgren E, Lundin-Olsson L, Håglin L, et al. High-intensity functional exercise program and protein-enriched energy supplement for older persons dependent in activities of daily living: a randomised controlled trial. Aust J Physiother. 2006;52(2):105‑13
90. Littbrand H, Rosendahl E, Lindelöf N, Lundin-Olsson L, Gustafson Y, Nyberg L. A high-intensity functional weight-bearing exercise program for older people dependent in activities of daily living and living in residential care facilities: evaluation of the applicability with focus on cognitive function. Phys Ther. 2006;86(4):489‑98.
91. Littbrand H, Lundin-Olsson L, Gustafson Y, Rosendahl E. The effect of a high-intensity functional exercise program on activities of daily living: a randomized controlled trial in residential care facilities. J Am Geriatr Soc. oct 2009;57(10):1741‑9.
92. Dechamps A, Diolez P, Thiaudière E, Tulon A, Onifade C, Vuong T, et al. Effects of exercise programs to prevent decline in health-related quality of life in highly deconditioned institutionalized elderly persons: a randomized controlled trial. Arch Intern Med. 25 janv 2010;170(2):162‑9
93. Faber MJ, Bosscher RJ, Chin A Paw MJ, van Wieringen PC. Effects of exercise programs on falls and mobility in frail and pre-frail older adults: A multicenter randomized controlled trial. Arch Phys Med Rehabil. juill 2006;87(7):885‑96.
94. Gallon D, Rodacki ALF, Hernandez SG, Drabovski B, Outi T, Bittencourt LR, et al. The effects of stretching on the flexibility, muscle performance and functionality of institutionalized older women. Braz J Med Biol Res. mars 2011;44(3):229‑35.
95. Grönstedt H, Frändin K, Bergland A, Helbostad JL, Granbo R, Puggaard L, et al. Effects of individually tailored physical and daily activities in nursing home residents on activities of daily living, physical performance and physical activity level: a randomized controlled trial. Gerontology. 2013;59(3):220‑9.
96. Hsu JK, Thibodeau R, Wong SJ, Zukiwsky D, Cecile S, Walton DM. A « Wii » bit of fun: the effects of adding Nintendo Wii(®) Bowling to a standard exercise regimen for residents of long-term care with upper extremity dysfunction. Physiother Theory Pract. avr 2011;27(3):185‑93.
97. Lazowski DA, Ecclestone NA, Myers AM, Paterson DH, Tudor-Locke C, Fitzgerald C, et al. A randomized outcome evaluation of group exercise programs in long-term care institutions. J Gerontol A Biol Sci Med Sci. 1999;54(12):M621‑8.
98. Lorenz RA, Gooneratne N, Cole CS, Kleban MH, Kalra GK, Richards KC. Exercise and social activity improve everyday function in long-term care residents. Am J Geriatr Psychiatry. 2012;20(6):468‑76
99. Makita M, Nakadaira H, Yamamoto M. Randomized controlled trial to evaluate effectiveness of exercise therapy (Takizawa Program) for frail elderly. Environ Health Prev Med. 2006;11(5):221‑7.
100. Sackley C, Wade DT, Mant D, Atkinson JC, Yudkin P, Cardoso K, et al. Cluster randomized pilot controlled trial of an occupational therapy intervention for residents with stroke in UK care homes. Stroke. 2006;37(9):2336‑41.
101. Sackley CM, Rodriguez NA, van den Berg M, Badger F, Wright C, Besemer J, et al. A phase II exploratory cluster randomized controlled trial of a group mobility training and staff education intervention to promote urinary continence in UK care homes. Clin Rehabil. 2008;22(8):714‑21.
102. Sackley CM, van den Berg ME, Lett K, Patel S, Hollands K, Wright CC, et al. Effects of a physiotherapy and occupational therapy intervention on mobility and activity in care home residents: a cluster randomised controlled trial. BMJ. 2009;339:b3123
103. Santana-Sosa E, Barriopedro MI, López-Mojares LM, Pérez M, Lucia A. Exercise training is beneficial for Alzheimer’s patients. Int J Sports Med. oct 2008;29(10):845‑50.
104. Seynnes O, Fiatarone Singh MA, Hue O, Pras P, Legros P, Bernard PL. Physiological and functional responses to low-moderate versus high-intensity progressive resistance training in frail elders. J Gerontol A Biol Sci Med Sci. 2004;59(5):503‑9.
105. Tak ECPM, van Hespen A, van Dommelen P, Hopman-Rock M. Does improved functional performance help to reduce urinary incontinence in institutionalized older women? A multicenter randomized clinical trial. BMC Geriatr. 2012;12:51.
106. Takeuchi R, Hatano Y, Yamasaki M. The influence of different exercise intervention programs on changes in quality of life and Activity of Daily Living levels among geriatric nursing home residents. J Phys Ther Sci. 2011;23:133-136.
107. Tappen RM. The effect of skill training on functional abilities of nursing home residents with dementia. Res Nurs Health. 1994;17(3):159‑65.
108. Tsaih P-L, Shih Y-L, Hu M-H. Low-intensity task-oriented exercise for ambulation-challenged residents in long-term care facilities: a randomized, controlled trial. Am J Phys Med Rehabil. 2012;91(7):616‑24.
109. Venturelli M, Scarsini R, Schena F. Six-month walking program changes cognitive and ADL performance in patients with Alzheimer. Am J Alzheimers Dis Other Demen. 2011;26(5):381‑8.
110. Robinovitch SN, Feldman F, Yang Y, Schonnop R, Leung PM, Sarraf T, et al. Video capture of the circumstances of falls in elderly people residing in long-term care: an observational study. Lancet. 2013;381(9860):47‑54.
111. Farlie MK, Robins L, Keating JL, Molloy E, Haines TP. Intensity of challenge to the balance system is not reported in the prescription of balance exercises in randomised trials: a systematic review. J Physiother. 2013 Dec;59(4):227–35.
112. Chen K-M, Li C-H, Chang Y-H, Huang H-T, Cheng Y-Y. An elastic band exercise program for older adults using wheelchairs in Taiwan nursing homes: a cluster randomized trial. Int J Nurs Stud. 2015;52(1):30–8
113. De Souto Barreto P, Demougeot L, Vellas B, Rolland Y. How much exercise are older adults living in nursing homes doing in daily life? A cross-sectional study. J Sports Sci. 2015;33(2):116‑24.
Supplementary materials
Search strategy performed on October 13th 2015
PubMed
1 ((adl[Title/Abstract]) OR «activity of daily living»[Title/Abstract]) OR «activities of daily living»[Title/Abstract]
2 (((«nursing home»[Title/Abstract]) OR «nursing homes»[Title/Abstract]) OR «long-term care facility») OR «long-term care facilities»
3 (((exercis*[Title/Abstract]) OR «physical activity»[Title/Abstract]) OR «physical therapy»[Title/Abstract]) OR «physical rehabilitation»[Title/Abstract]
4 («2011/08/01»[Date – Publication] : «3000»[Date – Publication])
5«randomized controlled trial»[Publication Type]
61 AND 2 AND 3 AND 4 AND 5
Cochrane Central Register of Controlled Trials
ID Search
#1 adl:ti,ab,kw or «activity of daily living»:ti,ab,kw or «activities of daily living» in Trials (Word variations have been searched)
#2 adl:ti,ab,kw or «activity of daily living»:ti,ab,kw or «activities of daily living»:ti,ab,kw in Trials (Word variations have been searched) – 5.554
#3 «long-term care facility»:ti,ab,kw or «long-term care facilities»:ti,ab,kw or «nursing home»:ti,ab,kw or «nursing homes»:ti,ab,kw (Word variations have been searched) – 2.410
#4 exercis*:ti,ab,kw or «physical activity»:ti,ab,kw or «physical therapy»:ti,ab,kw or «physical rehabilitation»:ti,ab,kw (Word variations have been searched) – 53.746
#5 «randomised clinical trial»:pt or «randomised clinical trials»:pt or «randomised control trial»:pt or «randomised control trials»:pt and «randomised controlled clinical trial»:pt (Word variations have been searched)
#6 «randomised clinical trial»:pt or «randomised control trial»:pt or «randomised controlled clinical trial»:pt or «randomised controlled study»:pt or «randomised controlled trial»:pt (Word variations have been searched) – 355.544
#7 #2 and #3 and #4 and #6
SportDiscus
S4 S1 AND S2 AND S3
S3TI exercis* OR AB exercis* OR TI «physical activity» OR AB «physical activity» OR TI «physical therapy» OR AB «physical therapy» OR TI «physical rehabilitation» OR AB «physical rehabilitation»
S2 TI «nursing home» OR AB «nursing home» OR TI «nursing homes» OR AB «nursing homes» OR TI «long-term care facility» OR AB «long-term care facility» OR TI «long-term care facilities» OR AB «long-term care facilities»
S1 TI adl OR AB adl OR TI «activity of daily living» OR AB «activity of daily living» OR TI «activities of daily living» OR AB «activities of daily living»
PsychInfo
S4 S1 AND S2 AND S3
S3 TI exercis* OR AB exercis* OR TI «physical activity» OR AB «physical activity» OR TI «physical therapy» OR AB «physical therapy» OR TI «physical rehabilitation» OR AB «physical rehabilitation»
S2 TI «nursing home» OR AB «nursing home» OR TI «nursing homes» OR AB «nursing homes» OR TI «long-term care facility» OR AB «long-term care facility» OR TI «long-term care facilities» OR AB «long-term care facilities»
S1 TI adl OR AB adl OR TI «activity of daily living» OR AB «activity of daily living» OR TI «activities of daily living» OR AB «activities of daily living»

