S. Gonfrier, S. Al Rifai, L. Benattar, S. Bernabé, O. Guérin
University Hospital of Nice, University of Nice Sophia Antipolis, CIU santé.
Corresponding author: Sebastien GONFRIER, University Hospital of Nice, University of Nice Sophia Antipolis, CIU santé, gonfrier.s@chu-nice.fr
Jour Nursing Home Res 2018;4:1-4
Published online January 9, 2018, http://dx.doi.org/10.14283/jnhrs.2018.1
Abstract
No study has been able to definitively demonstrate that it is effective at improving the behavior of patients with ADRS. The main objective of our study was to assess the influence of environmental light therapy (from 5 am to 10 pm) on nocturnal sleep patterns of individuals with ADRS. Secondary objectives were to study the time spent sleeping during the day, the level of anxiety based on the COVI scale, and the extent of behavioral disorders based on the NPI scale.Twelve nursing home residents were studied who were exposed to integrated light therapy in common areas. The residents were equipped with a wrist or ankle actimeter for 42 days. This time period was divided into three periods of 14 days, with standard lighting in period 1 and 3, and light therapy during period 2. Their sleep time was determined using Cole-Kripke algorithms. Neuropsychiatric symptoms were assessed based on the COVI scale for anxiety and the neuropsychiatric inventory (NPI).Duration of nocturnal sleep was significantly higher with light therapy by 15.8 minutes on average, the total sleep time during period 2 was significantly increased by 55.1 minutes compared to period 1. The COVI scale measurements indicated that there was a significant decrease of 0.7 points and the NPI scale decreased significantly by 4.9 points between periods 1 and 2. The use of environmental light therapy resulted in a significant increase in the nocturnal sleep and total sleep times, as well as improvement of the level of anxiety and in terms of behavior.
Key words: Dementia, nursing home, sleep, light therapy, behavioral disorders, Alzheimer’s disease, non pharmacological treatment.
Introduction
Alzheimer’s disease and related syndromes (ADRS) are diseases affecting memory, as well as the individual’s level of empowerment and their relationship with their environment. The WHO published a report on dementia and its management as a public health priority, both in terms of its prevalence and in terms of its economic impact on families, communities, and care systems. In 2014, the World Alzheimer Report estimates indicated that 35.6 million people worldwide were living with dementia. This number is expected to double by 2030 and to more than triple by 2050 (1). Of the various problems exhibited by patients with ADRS, behavioral disorders occur in 90% of cases. These include agitation, apathy, as well as secondary sleep disorders with an impairment of circadian rhythms (2). These circadian rhythm disorders are exhibited by 19 to 44% of patients with ADRS. The neurobiological basis for the latter is thought to be linked to degeneration of the suprachiasmatic nucleus. This entity is located in the hypothalamus, and it is involved in inhibition of melatonin secretion by light (3). In 2014, the Cochrane network published a systematic review of the literature for the past 20 years regarding the use of light therapy in patients with an ADRS. In total, 11 randomized controlled studies were included. These studies evaluated the effect of bright light generated by a lamp placed approximately 1 meter in front of patients for a duration ranging from 1 to 2 hours in the morning or at bedtime according to the various protocols that were employed. The primary endpoints of these studies focused on cognition, activities of daily life, sleep disorders, and behavioral disorders (4). On average, 45 patients (ranging from 13 to 94) were included in these 11 studies. Only one study was able to demonstrate a positive effect in six weeks and 2 years, but not at 1 year, in terms of improving the activities of daily life, although this could not be concluded definitively. The reasons given for the apparent lack of a clear effect of light therapy are the heterogeneity of the patients, the type of dementia, and the stage of the disease.
A study cited in the systematic review employed a randomized crossover methodology with an hour of light therapy versus standard lighting. Fifteen patients participated in the study. It was shown that sleep time increased significantly with light therapy. This study was not selected for the Cochrane systematic literature review because it had not been fully completed. Comparative results between each group nonetheless showed an effect on the sleep time of the 8 patients in the group that received the light therapy (5). All of the studies used elevated light intensities, ranging from 1,000 to 10,000 lux. The study that we conducted used a 400 lux light intensity, with a color change during the day to mimic the change in circadian brightness throughout the day. The aim of our study was to evaluate this environmental circadian lighting on the sleep patterns and behavior of patients with moderate to severe ADRS in a 12-bed sector compared to a fixed color light of the same intensity. This device was developed by TRILUX industrial.
Materials and methods
Population
Inclusion criteria were being over 60 years of age and being afflicted with moderate to severe ADRS, while also exhibiting any of the following: motor behavioral disorders, anxiety, restlessness, insomnia without underlying acute medical problems including no delirium. Non-inclusion criteria were acquired or congenital total blindness, being bedridden (i.e. loss of physical autonomy), and having resided in the nursing home for less than 15 days prior to the start of the experiment.
Light protocol and study design
The standard protocol consisted of fixed lighting with an illumination intensity of 400 lux and a fixed 5,100K color temperature. The experimental protocol consisted of dynamic circadian lighting, an illumination level of 400 lux with a range of variation according to the circadian rhythm of the color temperature from 3,000 to 6,500K that was smoothened from 6 am to 10 pm. The light was turned off form 10 pm to 6 am. Due to limitations placed of the study (e.g. only one area with 12 beds could be equipped with the circadian lighting system), we choose to use the subjects as their own controls, as addition of separate controls was not feasible. To control the time period, we used an experimental protocol comprised of three 14 day periods. Standard lighting was used during the first and the third period, and circadian lighting was used during the second period. It was a pilot intra-individual comparative study that was approved by an independent ethics board.
Evaluation criteria
Actimetry
The primary endpoints were sleep time as assessed by actigraphy (ActiGraph™ tri-axis accelerometer monitor GT3X+) between 10 pm and 5 am using Cole-Kriple algorithms (6). Secondary endpoints were total sleep time over a 24 hour period and the number of intra-awakening sleeps. Actimetry watches were worn continually on the non-dominant wrist or at the ankle when the wrist was not a suitable option, after a 24h adjustment period prior to the start of the study.
Behavioral scale
Behavioral disorders were measured every day during a meeting with staff that was directed by a trained psychologist using the NPI-ES (7) scale, and anxiety levels were measured by using the COVI scale (8).
Data collection and analysis
Data collection for the behavioral scales was carried out by psychologists from the nursing home. The two psychologists in question were experienced in management of residents with ADRS.
For actimetry, the recording was continuous. Data were saved and actimetry watches were reloaded between each period.
Study data
The data analysis was by intention to treat. A control of data quality had previously been achieved. The analysis of the overall data was performed by mixed linear regression model methods. The period was considered a fixed effect and the residents as a random effect. A post-hoc analysis with multiple comparisons by the Bonferroni (9) method was performed when differences between periods were found. All analyses were performed using SPSS 22.0 software. The significance level was set at 5%.
Results
The population was comprised of 5 men and 7 women who were 70 to 92 years of age (mean of 84.2; SD 12.7). The likely ADRS presented by the patients were severe Alzheimer’s disease stage for 7 residents, one case of intermediate Alzheimer’s disease stage, three cases of severe mixed Alzheimer’s disease and vascular dementia, and one case of intermediate stage vascular disease.
The following table 1 and figure 1 show the overall results of the study. Nocturnal sleep time (i.e. from 10 pm to 5 am) exhibited a significant difference in duration of 15.8 minutes. Total sleep increased significantly by 55.1 minutes, and the mean number of nocturnal awakenings decreased by 1.1 between period 1 and period 2. In terms of the daytime parameters, the behavior scales revealed a significant reduction in anxiety (-0.7 points on the COVI scale) and a significant improvement in behavioral disorders (-4.9 points on the NPI score). The difference between period 2 and period 3 did not reach significance, except for the number of nocturnal awakenings, for which the mean decreased by 1.4.
*Significance of the period in a linear mixed model adjusted based on the patient; ** Significance of the differences between periods in a linear mixed model adjusted based on the patient with Bonferroni comparison
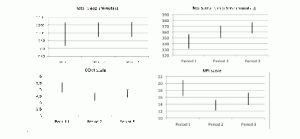
Figure 1
Representation of mean with confidence interval of total sleep, nocturnal sleep time, COVI scale and NPI scale by period
Discussion
We were able to demonstrate an improvement both in terms of sleep times and behavioral disorders between standard and circadian lighting in the first two periods. This improvement did not regress after returning the residents to standard lighting in period three.
The study involved 12 participants, which represent a significant limitation. However, each participant in this study acted as their own control. This provided a good comparison control, thus reducing the heterogeneity of the measurements. Before the start of the study, each resident was subjected to circadian lighting for more than 15 days, as sudden changes in lighting have been shown to cause sleep disturbances in residents. By examining the standard deviation of the data, it can be seen that it was pronounced for the first period and that it became less pronounced for the next two periods. It would appear that the transition from the experimental to the standard lighting caused a substantial degree of fluctuation in sleep, while it increased during the experimental period. The two lightings differed in terms of their color compositions, while the intensity remained the same at 400 lux. Between the first and the second period, the statistical model that was used revealed a significant improvement in the key parameters that were measured to determine the duration of nocturnal sleep, total sleep time, as well as the level of anxiety and the extent of behavioral disorders.
The change of lighting between the second and the third period revealed no change other than persistently improved sleep. The duration (15 days) had been determined empirically on the basis of previously conducted experimental studies. Chronobiology is a phenomenon that changes gradually. It is possible that the absence of sleep changes during the third period is a residual effect of the light therapy in period 2. A Norwegian study of 11 nursing home patients equipped with actimeters who were exposed to experimental light therapy intensities between 6,000 and 8,000 lux for 2 hours per day over a 14 day period exhibited improved sleep behaviors as well as persistence of this improvement one month after cessation of the light exposure (10). This study hence showed that the residual nature can have a therapeutic effect that may explain the failure to respond to cessation of light exposure in the 3rd period. Another explanation for this may lie with the effect of the chronological sequence, as the study started on October 26, 2015 and ended on December 8, 2015, during which time days in fact become progressively shorter. Even if the residents did not go outside for any length of time, and the lighting was more intense inside the establishment, the days becoming shorter may have nonetheless influenced their sleep patterns, thus explaining the persistence of the effect on nocturnal sleep duration in the third period. To accommodate for this, a crossover study ought to have been performed, with randomization of two structures at the same time, which was impossible with the initial prerequisites.
Another structural bias is that the devices were present in the common areas, but not in the residents’ individual rooms. A resident who remained in their room throughout the day would hence not have been exposed to the light therapy. Moreover, since movement throughout the facility is not restricted, some residents may have spent a disproportionate amount of time in the non-equipped section of the facility.
Not all of the residents could wear the watch actimeters on their wrists. In fact, only five residents were able to wear them on their wrists, the others having to forego this option despite a strong attachment, thus prompting us to instruct them to wear the devices on their ankles.
Regarding the psychometric scales, we found the same trend, namely improved behavior both in terms of anxiety and general behavior disorders. The concordance of an effect on sleep and both forms of behavior is a causal argument linking the lighting change with behavioral changes of the residents, even though reversibility could not be demonstrated in this study.
According to a meta-analysis published in 2016 by Van Maanen A et al (11), who analyzed 11 studies involving patients with ADRS, the effect of light therapy on total sleep duration was significant, although it still only amounted to a small effect (Hedges’ g statistic, g = 0.28). Compared to this study, measuring sleep by actigraphy seems like a more objective approach. A study evaluating recommendations for studies using light therapy in nursing homes recommend combined use of actigraphy and clinical observations, as we have done (12). Otherwise, the light therapy used in this study was an integrated therapy in the facility that the patients were in fact exposed to from 5 am to 10 pm, or a maximum of 17h. The novelty of this work lies with the fact that this therapeutic method is innovative and not strictly comparable with studies using fixed devices and an exposure of between 1 and 2 hours at different times of the day with an average intensity of 4,400 lux (ranging from 1,000 to 10,000 lux).
In the literature review from the Cochrane Library in 2014, the 11 selected studies revealed no effect on behavior (4). The heterogeneity of disorders among individuals and groups that were compared is thought to underlie the lack of significance in the differences. A study published in the JAGS in November 2007 by Hickman et al. (13) demonstrated an effect of intense light on depression. Indeed the effect of four types of lighting on the moods of 66 subjects exposed to 2500 Lux in two separate structure crossovers for either 4 hours in the morning (7 am-11am), 4 hours in the afternoon (4 pm-8pm), all day (7 am-8pm), or no stimulation was investigated. The default condition without stimulation was 600 lux of intensity. The outcome was based on a scale for measuring depression (the Cornell Scale for Depression in Dementia, CSDD). The results showed that the high intensity light had a positive effect on depressive symptoms in women, but a deleterious effect on depressive symptoms in men. The high brightness sequence was most effective in the morning. This study reinforces the notion of relying on the color of the light rather than the intensity to improve behavioral disorders.
Conclusion
Our study, despite its various biases, was able to discern an influence of light on the sleep patterns and the behavior of residents who had not previously been exposed to a circadian lighting system. To conclusively demonstrate such an effect would require a comparative simultaneous crossover study in two areas. This could be achieved if at least two areas were equipped with the same lighting devices. In view of the greater variability in the first period and the residual effect in the third period, the crossover study could be conducted over a longer period of time (e.g. 28 days). According to some studies (14-16), variability of sleep involves more than the circadian cycle. It has been noted that there may be an effect of the lunar cycle on the sleep patterns of healthy individuals. This could interfere with the timing of our study as the lunar cycle is 28 days. A protocol comparing crossover residents keeping the same measurements over two periods of 28 days could, with a more robust methodology and ideally using only wrist actimeters, help establish the effect of circadian light on the sleep patterns and the behavior of individuals with ADRS.
Conflicts of Interest: ORPEA Group and TRILUX Group financially support the study
References
1. WHO-Dementia-English.pdf [Internet]. [cited 16 Aug 2016]. Disponible sur: https://extranet.who.int/agefriendlyworld/wp-content/uploads/2014/06/WHO-Dementia-English.pdf
2. Steinberg M, Shao H, Zandi P, Lyketsos CG, Welsh-Bohmer KA, Norton MC, et al. Point and 5-year period prevalence of neuropsychiatric symptoms in dementia: the Cache County Study. Int J Geriatr Psychiatry. Febr 2008;23(2):170 7.
3. McCurry SM, Reynolds CF, Ancoli-Israel S, Teri L, Vitiello MV. Treatment of sleep disturbance in Alzheimer’s disease. Sleep Med Rev. Dec 2000;4(6):603 28.
4. Forbes D, Blake CM, Thiessen EJ, Peacock S, Hawranik P. Light therapy for improving cognition, activities of daily living, sleep, challenging behaviour, and psychiatric disturbances in dementia. Cochrane Database Syst Rev. 2014;(2):CD003946.
5. Lyketsos CG, Lindell Veiel L, Baker A, Steele C. A randomized, controlled trial of bright light therapy for agitated behaviors in dementia patients residing in long-term care. Int J Geriatr Psychiatry. Jul 1999;14(7):520 5.
6. Cole RJ, Kripke DF, Gruen W, Mullaney DJ, Gillin JC. Automatic sleep/wake identification from wrist activity. Sleep. Oct 1992;15(5):461 9.
7. Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. Dec 1994;44(12):2308 14.
8. Lipman RS, Covi L. Outpatient treatment of neurotic depression: medication and group psychotherapy. Proc Annu Meet Am Psychopathol Assoc. 1976;(64):178 218.
9. Bland JM, Altman DG. Multiple significance tests: the Bonferroni method. BMJ. 21 Jan 1995;310(6973):170.
10. Fetveit A, Bjorvatn B. The effects of bright-light therapy on actigraphical measured sleep last for several weeks post-treatment. A study in a nursing home population. J Sleep Res. Jun 2004;13(2):153 8.
11. Van Maanen A, Meijer AM, van der Heijden KB, Oort FJ. The effects of light therapy on sleep problems: A systematic review and meta-analysis. Sleep Med Rev. 9 Sep 2015;29:52 62.
12. Van der Ploeg ES, O’Connor DW. Methodological challenges in studies of bright light therapy to treat sleep disorders in nursing home residents with dementia. Psychiatry Clin Neurosci. Nov 2014;68(11):777 84.
13. Hickman SE, Barrick AL, Williams CS, Zimmerman S, Connell BR, Preisser JS, et al. The effect of ambient bright light therapy on depressive symptoms in persons with dementia. J Am Geriatr Soc. Nov 2007;55(11):1817 24.
14. Della Monica C, Atzori G, Dijk D-J. Effects of lunar phase on sleep in men and women in Surrey. J Sleep Res. Dec 2015;24(6):687 94.
15. Cajochen C, Altanay-Ekici S, Münch M, Frey S, Knoblauch V, Wirz-Justice A. Evidence that the lunar cycle influences human sleep. Curr Biol. 5 Aug 2013;23(15):1485 8.
16. Sjödin A, Hjorth MF, Damsgaard CT, Ritz C, Astrup A, Michaelsen KF. Physical activity, sleep duration and metabolic health in children fluctuate with the lunar cycle: science behind the myth. Clin Obes. Apr 2015;5(2):60 6.

