I. Røen1, J. Šaltytė Benth1,2,3 , Ø. Kirkevold1,4,5, I. Testad6,7,8, G. Selbæk1,4,9, K. Engedal4, S. Bergh1,4
1. Centre for Old Age Psychiatric Research, Innlandet Hospital Trust, Ottestad, Norway; 2. Institute of Clinical Medicine, Campus Ahus, University of Oslo, Oslo, Norway; 3. Health Services Research Unit, Akershus University Hospital, Lørenskog, Norway; 4. Norwegian National Advisory Unit on Ageing and Health, Vestfold Hospital Trust, Tønsberg, Norway; 5. Norwegian University of Science and Technology (NTNU), Department of Health Science in Gjøvik, Gjøvik, Norway; 6. Centre for Age-Related Medicine – SESAM, Stavanger University Hospital, Stavanger, Norway; 7. Department of Old Age Psychiatry, Institute of Psychiatry, Psychology & Neuroscience, King’s College London, London, UK; 8. University of Exeter Medical School, St Luke’s Campus, University of Exeter, UK. 9. Faculty of Medicine, University of Oslo, Oslo, Norway. Corresponding author: Irene Røen, Centre for Old Age Psychiatric Research, Innlandet Hospital Trust, p.b. 68, 2312 Ottestad, Norway. E-mail address: irroee@sykehuset-innlandet.no, Phone: +47 90652165
Jour Nursing Home Res 2019;5:8-19
Published online Februay 11, 2019, http://dx.doi.org/10.14283/jnhrs.2019.2
Abstract
Background: There is no cure for dementia and appropriate care should be offered to improve or maintain quality of life for those living with dementia. Objectives: To identify groups of residents following similar trajectories of quality of life after nursing home admission, to examine which resident, staff, and organizational characteristics at baseline differ between the identified groups, and to assess the associations between the trend in quality of life and the same characteristics measured at baseline and over the study period. Design: A prospective, observational, longitudinal cohort design over 30 months. Setting: Nursing homes in Norway. Participants: Residents admitted to nursing homes. Measurements: Resident data on quality of life, dementia, pain, activities of daily living, physical health, neuropsychiatric symptoms, medication, and demographic characteristics were obtained by interviews. Unit characteristics and the staff data on person-centered care, psychosocial factors, and job satisfaction were obtained by questionnaires and interviews. The physical environment of the unit was assessed by structured observation. Results: 694 residents admitted to a nursing home and 1161 staff from 175 nursing home units participated. Three resident groups following similar trajectories in quality of life were identified by growth mixture model; good quality of life (53.6%), moderate quality of life (32.9%), and poor quality of life (13.4%). All groups’ quality of life decreased over time. More pain, more severe dementia, and more affective symptoms at baseline were associated with belonging to the poor quality of life group. Overall decline in quality of life was associated with more severe dementia, more pain, poorer function in activities of daily living, more severe neuropsychiatric symptoms among residents, and poorer job satisfaction among staff. Conclusion: Reducing pain, reducing NPS, improving activities of daily living for the residents, and improving the staff’s job satisfaction may be factors of importance to improve the residents’ quality of life.
Key words: Quality of life trajectories, nursing home, dementia, job satisfaction, physical environment.
Introduction
Dementia is a chronic syndrome that can be caused by a variety of brain disorders, most frequently Alzheimer’s disease. It is characterized by cognitive decline, impaired functioning in daily life activities, deterioration of emotional control, and change of social behavior or motivation, and is one of the factors most strongly associated with admission to a nursing home (NH) (1).
There is no cure for any of the brain disorders causing dementia, and appropriate care should be offered to improve or maintain the quality of life (QoL) of persons with dementia (2). QoL is a multidimensional concept encompassing the emotional, physical, social, and environmental domains of a person’s wellbeing (3). Several studies have investigated QoL in NH patients, both self reported and proxy reported (family and staff), where age, ADL, dementia severity, pain, psychiatric disorders, pulmonary diseases and neuropsychiatric symptoms (NPS), are found to be associated with reduced QoL in NH patients with dementia (4, 5). Previous studies, following QoL in persons with dementia over time in NH, found QoL to be rather stable (6-8). Attention towards improving quality of NH care and QoL for NH residents is needed, and staff knowledge and skills should be reinforced to maintain or improve the residents’ QoL. A review from 2016 found that when the staff were trained to interact empathetically and humanely with the patients in their care, the residents experienced fewer depressive symptoms, less functional dependence, better food intake, and less psychotropic medications were prescribed. The review concluded that associations exist between potentially adjustable staff variables and Quality of care onwards to QoL (9).
Person-centered care (PCC) is regarded as good quality of care and is a guiding principle in dementia care (10, 11). An increasing amount of literature has evaluated resident outcomes of PCC, showing significant benefits such as decrease in NPS, reduced prescription of psychotropic medication, improved mood, improved QoL, and cost-effectiveness in providing care to persons with dementia in long-term care (11, 12).
Additionally, a recent literature review concluded that the physical environment of care settings is important in improving the residents’ QoL and in improving quality of care practices (13).
The previous longitudinal studies on QoL in nursing homes (6-8) have small cohort size, follow their participants over a short period of time, and to a lesser extent investigate associations with important patient, staff- and NH-variables. Therefore, we designed a study to identify groups of residents following similar trajectories of QoL after admission to NH, over a period of 30 months; and, to examine how resident, NH staff, and unit characteristics measured at baseline were associated with the group-belonging. Additionally, we aimed at assessing the associations between the same characteristics and the overall trend in QoL, with the characteristics measured simultaneously as QoL whenever possible.
Methods
Design
This is a longitudinal observational study of patients in 47 NHs in Norway, previously described in the “Resource Use and Disease Course in dementia – Nursing Home (REDIC-NH) study” (14). Resident baseline data were collected at admission to the NH (within one month of admission), and follow-up data were collected biannually for 30 months. The baseline data were collected between March 2012 and November 2014, and the last follow-up data were collected in May 2017.
NH demographics and staff characteristics were obtained through questionnaires distributed to the staff and the head nurse of the NH units; these included both standardized questionnaires and questions developed for this study by the reseach group. The members of the research group are highly experienced in both clinical practice and NH research, and the questions developed were based on previous experience, and literature regarding organizational and structural factors in NH (i.e. staff level and education, leadership, management, physical environment and culture). The physical environment of the units was assessed by structured observation between October 2013 and December 2014, presented in a previous study (15).
Ethics approval and consent to participate
The residents’ capacity to consent to participate in the study was evaluated by the NH staff and physician, in close collaboration with relatives. Written consent for participation was obtained from all participants with the capacity to consent, and for participants lacking the capacity to consent, the next of kin gave consent on behalf of the residents. Data from the staff and the head nurses were collected anonymously.The Regional Ethics Committee for Medical Research in South-Eastern Norway approved the study (2011/1738a).
Participant inclusion criteria
Nursing home resident
All residents 65 years or older, regardless of degree of cognitive function, were eligible for inclusion in the study. In addition, we included residents younger than 65 years with established dementia, as their symptoms and functional decline over time resembles elderly patients in NH. Residents with an expected stay in the NH under four weeks or an expected life expectancy of less than six weeks were excluded.
Nursing home staff
NH staff who knew the residents in the unit and the organizational structure of the unit well, were eligible for inclusion in the study.
Sample characteristics and measurements
Nursing home residents
Demographic characteristics of the residents included age, gender, marital status, and medication. The residents’ QoL was assessed with the Quality of life in Late-Stage Dementia scale (QUALID), a standardized and validated proxy-based questionnaire with a sum score ranging from 11 to 55, with lower scores indicating a better QoL (16). QUALID is not validated for persons without dementia, but as the aim of the study was to follow the participants over 30 months we expected that some of the participants without dementia at baseline, would develop dementia during the study period. In addition, some participants would have severe dementia and a proxy-based QoL assessment tool would be useful. Therefore, we judged QUALID a sensible assessment tool in our study. Dementia at baseline was diagnosed independently by GS, KE and SB using all available collected information. In case of disagreement the cases were discussed until consensus was reached. Dementia severity was assessed with the Clinical Dementia Rating scale (CDR), a global rating scale covering six domains of cognitive and functional performance (17). The CDR sum of boxes (CDR-SOB) was calculated by adding the domain scores, which range from 0 – 18, with a higher score indicating more severe dementia (18). Pain was assessed with the Mobilization-Observation-Behavior-Intensity-Dementia Pain Scale (MOBID-2), which consists of 10 items, each item score ranging from 0 to 10, with a higher score indicating more severe pain (19). ADL function was assessed with the Physical Self-Maintenance Scale (PSMS), a six-item scale ranging from 6 to 30, with a higher score indicating a lower level of functioning (20). General physical health was assessed using the General Medical Health Rating (GMHR) scale, a one-item global rating scale with four categories (excellent, good, fair, poor) (21). Neuropsychiatric symptoms (NPS) were assessed using the 12-item Neuropsychiatric Inventory nursing home version (NPI-NH) (22). An NPI item score was calculated by multiplying frequency (0-4) with severity (0-3), producing an item score (0-12), with a higher score indicating more severe NPS. NPI sub-syndrome scores were calculated based on a previous factor analysis: NPI agitation (agitation/aggression, disinhibition, and irritability, range 0-36), NPI psychosis (delusions and hallucinations, range 0-24), and NPI affective (depression and anxiety, range 0-24) (23).
Nursing home staff
NH staff characteristics were obtained through questionnaires, and included age, gender, Norwegian as first language, number of years of health-related education, experience in the current job, and percentage of full-time position.
Person-centered care was assessed with the Person-centered Care Assessment Tool (P-CAT), consisting of 13 items that are formulated to measure staff perceptions of the practice in the unit where they work. The total score ranges from 13 to 65, with higher scores indicating a higher level of PCC (24).
Work-related psychosocial factors were assessed with 32 of the 129 items in the General Nordic Questionnaire for Psychosocial and Social Factors at Work (QPS-Nordic), covering essential social and psychological factors at work (25). The 32 items are distributed across 10 scales; each scale consists of 3 or 4 items, giving a subscale score of 3-15 or 4-20, with higher scores indicating better work-related psychosocial factors.
NH staff’s job satisfaction was measured with a single question: “How would you describe your general experience of your job satisfaction?” with seven possible answers: “very bad – bad – unsure – quite good – good – excellent”.
Unit characteristics
A unit in a NH was defined as a group of residents who live together with a common living area and who have their own care staff during the daytime. Data were collected about the unit size (number of residents); the daytime staff/resident ratio (the number of NH staff working per resident during the daytime); the type of unit (special care unit [SCU] or regular unit [RU]); the number of hours the nursing home physician was working per resident per week; the number of units per head nurse; and whether the unit had a nursing professional development specialist.
The physical environment of the unit was assessed with the Special Care Unit Environmental Quality Scale (SCUEQS), a summary scale embedded in the Therapeutic Environment Screening Survey for Nursing Homes (TESS-NH) (26). Scores range from 0 – 41, with higher scores indicating a better physical environment.
Statistical methods
Resident, NH staff, and unit characteristics were described as means and standard deviations (SD) or as frequencies and percentages. Participants vs. non-participants were compared by Independent samples t-test or χ2-test. Staff characteristics used in regression models were aggregated to a mean score at each unit. Missing values on staff-rated person-centered care (P-CAT) items were imputed on cases with fewer than 50% missing on the P-CAT scale by generating an empirical distribution for each item and drawing a random number from it. As an exploratory approach, growth mixture model was estimated to identify potential groups of residents that were following similar trajectories in QoL score throughout the study period. According to this method, the groups are identified based on individual trajectories of residents. The number of groups was determined by applying Akaike’s Information Criterion (AIC), where a smaller value indicates a better model. It was also required, that 95% confidence intervals of trajectories were non-overlapping and within-group probabilities high, preferably 0.80 or higher. Nominal regression analysis was performed to assess if resident and NH staff characteristics and/or factors related to the physical environment (as measured at baseline) were associated with group-belonging. No clustering within the NH-unit was present, and hence no adjustment was implemented into the nominal regression model. The associations between the same characteristics as measured at baseline or all assessments (whenever available) and the overall trend in QoL were assessed by estimating a linear mixed model with random effects for residents, units, and the interaction between the two. The fixed effects for time coded as dummies and for characteristics were included. The multiple models were further reduced by AIC. Regression models were estimated on cases with no missing values on covariates.
Statistical analyses were performed in IBM SPSS V25 and SAS 9.4. Results with p-values below 0.05 were considered statistically significant.
Results
Six-hundred and ninety-four residents and 1161 NH staff from 175 NH units from 47 NH, from 4 counties in Norway were included in the study.
To compare age and gender of participants vs. non- participants, 38 of the 47 NH collected data on all residents eligible for inclusion. Of 1331 eligible residents in the 38 NH, 607 were included and 724 did not participate (205 declined participation, 191 died before inclusion took place, and 328 was not included for unknown reasons). The mean age of participants was 84.4 years (SD 7.5), while for non-participants it was 83.6 years (SD 9.3) (p = 0.048); 64.4% of participants and 56.6% of non-participants were women (p = 0.004).
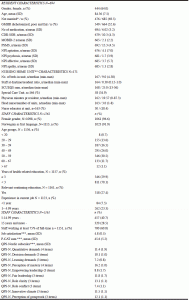
Table 1 presents resident, unit, and staff characteristics at baseline. The residents had a mean age of 84.4 years, 64% were women, 52.6% had poor/fair physical health, and the mean CDR-SOB was 10.3.
N varies between variables due to missing data; * not married; including singles, widowed, and residents divorced or separated opposed to married; including residents being married or living with a partner; ** Nursing home unit was defined as a group of residents living together with a common living area and having their own care staff during the daytime; *** Staff variables aggregated at unit n= 160/175; SD= standard deviation; GMHR= General Medical Health Rating Scale (excellent, good, fair, poor); CDR-SOB= Clinical Dementia Rating Scale sum of boxes (range 0 – 18); MOBID-2= Mobilization-Observation-Behavior-Intensity-Dementia Pain Scale (range 0 – 10); PSMS= Physical Self-Maintenance Scale (range 6 – 30); NPI agitation= sum of agitation/aggression, disinhibition, and irritability (range 0 – 36); NPI psychosis= sum of delusions and hallucinations (range 0 – 24); NPI Affective= sum of depression and anxiety (range 0 – 24); SCUEQS= Special Care Unit Environmental Quality Scale (range 0 – 41); P-CAT= Person-centered Care Assessment Tool (range 13-65); QPS-Nordic= General Nordic Questionnaire for Psychosocial and Social Factors at Work; QPS-Nordic subscales each consist of 3 or 4 items (range 3-15 or 4-20)
QUALID: Quality of life in Late-Stage dementia scale, ranging from 11 to 55
Trajectories of QoL
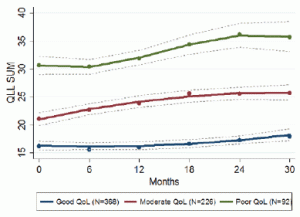
Eight residents were excluded from the growth mixture model due to missing baseline QoL data, leaving 686 residents for the analysis. Three QoL groups of residents following similar trajectories were identified (Table 2 and figure 1). All three groups exhibited a decline in QoL over 30 months. The group with best QoL named “good QoL” (n = 368, 53.6%) experienced a small deterioration in QoL from 16.1 (see intercept table 2) to 18.2 points at 30-month follow-up. Another group named “moderate QoL” (n = 226, 32.9%) experienced a deterioration in QoL from 21.0 to 25.5 points at 30-month follow-up. The last group, named “poor QoL” (n = 92, 13.4%), experienced a stable QoL, from 30.6 to 31.5 points, during the first 24 months, and thereafter remaining stable until 30 months. Table 2 presents the demographic and clinical characteristics within the three groups.
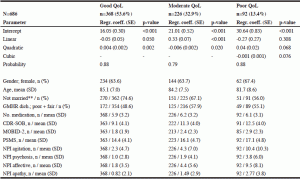
Table 2
Parameter estimates of growth mixture model and residents’ characteristics within three groups of QoL *
* only cases with no missing item at baseline QUALID sum included; ** not married; including singles, widowed, and residents divorced or separated opposed to married; including residents being married or living with a partner; QoL= Quality of Life; SD= standard deviation; GMHR= General Medical Health Rating Scale (excellent, good, fair, poor); CDR-SOB= Clinical Dementia Rating Scale sum of boxes (range 0 – 18); MOBID-2= Mobilization-Observation-Behavior-Intensity-Dementia Pain Scale (range 0 – 10); PSMS= Physical Self-Maintenance Scale (range 6 – 30); NPI agitation= sum of agitation/aggression, disinhibition, and irritability (range 0 – 36); NPI psychosis= sum of delusions and hallucinations (range 0 – 24); NPI Affective= sum of depression and anxiety (range 0 – 24)
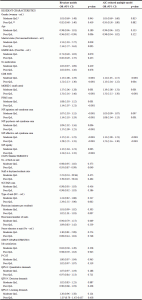
Table 3 presents the results of the nominal regression model, with group-belonging as the outcome variable and characteristics measured at baseline as explanatory variables. In the AIC-reduced multiple model, more severe dementia, more pain (MOBID-2), and more severe affective symptoms at baseline were associated with higher odds of belonging to the group named moderat QoL and poor QoL groups compared to the group named good QoL, while more severe symptoms of agitation at baseline was associated with higher odds of belonging to the group named poor QoL as compared to the group named good QoL.
Good QoL group as reference. N=561 (N=305 in good QoL group, N=182 in moderate QoL group, N=74 in poor QoL group), cases with at least one missing value on covariates were excluded
Variables associated with QUALID score over time
Table 4 presents the results of the linear mixed model for the associations between the residents’ QUALID score and the resident, staff, and unit characteristics measured simultaneously or, if not possible, at baseline. More severe dementia, more pain, lower ADL-function, and more severe NPS (except for the NPI psychosis sub-syndrome) were associated with decreasing QoL in the AIC-reduced multiple model. Better job satisfaction among the staff was associated with increasing resident QoL during the observation period.
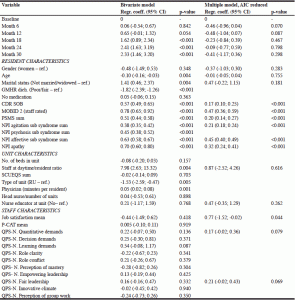
Table 4
Linear mixed model with QoL (QUALID score, ranging from 11 to 55) sum as dependent variable
(N=1942, based on the number of residents assessed with QUALID at the six time points).
Cases with at least one missing value on covariates were excluded
GMHR= General Medical Health Rating Scale (excellent, good, fair, poor); CDR-SOB= Clinical Dementia Rating Scale sum of boxes (range 0 – 18); MOBID-2= Mobilization-Observation-Behavior-Intensity-Dementia Pain Scale (range 0 – 10); PSMS= Physical Self-Maintenance Scale (range 6 – 30); NPI agitation= sum of agitation/aggression, disinhibition, and irritability (range 0 – 36); NPI psychosis= sum of delusions and hallucinations (range 0 – 24); NPI Affective= sum of depression and anxiety (range 0 – 24); SCUEQS= Special Care Unit Environmental Quality Scale (range 0 – 41); P-CAT= Person-centered Care Assessment Tool (range 13-65); QPS-Nordic= General Nordic Questionnaire for Psychosocial and Social Factors at Work; QPS-Nordic subscales each consist of 3 or 4 items (range 3-15 or 4-20)
Discussion
In the present study, we followed 694 nursing home residents for 30 months after admission to a nursing home. Three resident groups following distinct trajectories in QoL, as measured with the QUALID sum score, were identified. The majority of the residents belonged to the group named good QoL over time. This group and the group named poor QoL had the most stable QoL through the study period, although all three groups exhibited some decline in QoL. Furthermore, the study showed that more severe dementia, more pain, poorer ADL-function, and more severe NPS, as assessed throughout the study period, were associated with an overall decrease in QoL.
Several studies have shown an association between different resident characteristics and QoL (27, 28). The pattern is clear: more severe dementia, poorer ADL function, and more severe NPS—especially more severe depression and anxiety symptoms—are all associated with worse QoL (29). Our results are in line with these previous findings. Thus, to improve the residents’ QoL, it is important to treat or prevent pain and NPS, especially depression and anxiety, and to improve or maintain ADL function.
We found that higher job satisfaction among the staff at baseline was associated with an overall increase in the residents’ QoL. Previous studies have reported that job satisfaction is associated with quality of care (30), but to our knowledge no other studies have investigated the influence of staff job satisfaction on residents’ QoL. This is an important finding, underpinning the urgency of focusing on job satisfaction and retention of NH staff to meet the increasing need for qualified NH staff, due to an aging society. We expected that a higher level of PCC would improve the residents’ QoL, as a guiding principle for good quality of care (10, 11), but found no such association in the present study. However, in a previous study based on the same data, we found that the staff’s job satisfaction was positively associated with PCC (15), and efforts to improve the staff’s job satisfaction may improve both the PCC given at the ward and the residents’ QoL.
The importance of the physical environment is increasingly recognized as a therapeutic resource in NH (13). However, the present study was not able to demonstrate an association between the physical environment and the residents’ QoL. This may be due to the homogeneity between NH units included in the study, the characteristics of the assessment tool used, or the sample size. However, four staff and unit characteristics (staff at daytime/resident ratio, nurse educator at unit, QPS-N quantitative demands and fair leadership) were kept in the AIC-reduced model as important covariates, indicating that this factors contribute to the better model fit.
Interestingly, except for the staff’s job satisfaction, the only characteristics associated with the residents’ QoL were individual resident characteristics. This association was found both in the growth mixture and the linear mixed models, which strengthens the result. Other studies have demonstrated that there are associations between staff variables and the quality of care, influencing the resident’s QoL (9). Studies have also shown that PCC are positively associated with the staff’s job satisfaction (30), which may improve NPS in persons with dementia and can reduce NPS in people with dementia living in NH (12). Improving ADL function would be benefitical for the patients and improving their QoL.
Strengths and limitations
A strength of the study is that residents are included at admission to the NH, and the longitudinal follow-up design of the study with analysis of the residents’ characteristics and QoL at the same time points. The linear mixed model analysis captures how the residents’ characteristics change over the follow-up period simultaneously with their QoL changes. The same characteristics associated with poor QoL were found for both baseline characteristics and longitudinally assessed characteristics, and the two independent analyses confirming the same associations istrengthens the result.
There are several limitations in the study. The complex causal pathways between unit characteristics, staff characteristics, and resident characteristics are a limitation when designing a study that investigates which factors are associated with the residents’ QoL. Another limitation is the fact that the residents did not rate their own QoL; rather, we had to rely on proxy report by the NH staff, which could possibly lead to biased data. When proxies rate a person as having reduced function in ADL and NPS, they may also assume that the person’s QoL is reduced. It is reported that people with dementia rate their QoL higher than proxies do (2). However, the proxies assessed the residents’ QoL with a standardized and validated questionnaire, that is used internationally to rate QoL, which should help to reduce the subjectivity in the rating.
Patient characteristics were assessed every six month, but units and staff characteristics were assessed only once, at baseline, which is a limitation in the study. While the unit characteristics would be rather stable over the follow-up period, staff characteristics could change due to increased or decreased knowledge or new staff recruiting.
Finally, limitations due to the data collection procedure should be noted, as a high number of project nurses collected data on resident characteristics, which could in turn excess heterogeneity of the data. Furthermore, the study cohort may not accurately represent the general NH population, as information from 38 of the 47 NH revealed that there were more women in the included-residents group than in the eligible-but-not-included residents group (14, 15).
Conclusion/relevance
Overall, the majority of residents belonged to the group named good QoL over the observation period of 30 months. Residents in the group named poor QoL was associated with having more pain, more severe dementia, more affective symptoms, and living in a unit with poorer staff job satisfaction at baseline, as well as more pain, poorer ADL function, and more severe NPS measured simultaneously. As dementia is a chronic disease, focus on symptom relief and QoL is important. Efforts that focus on reducing pain, reducing NPS, and improving ADL function for the resident, as well as improving the job satisfaction of the staff may be important factors to improve resident QoL.
Conflict of interest: None.
Description of authors’ roles: I. Røen designed the study, collected the data and wrote the paper. J. Šaltytė Benth performed the statistical analysis and assisted with writing the paper. Ø. Kirkevold was involved in designing the study and in the statistical analysis, and assisted in writing the paper. I. Testad critically revised the manuscript. G. Selbæk was involved in designing the study and critically revised the manuscript. K. Engedal critically revised the manuscript. S. Bergh was involved in designing the study, supervised the data collection and assisted with writing the paper. All authors read and approved the final version of the manuscript.
Funding: The Norwegian Health Directorate provided funding for the data collection. The first author’s Ph. D. study was funded by the Research Counsil of Norway.The sponsors had no role in the design and conduct of the study; in the collection, analysis, and interpretation of data; in the preparation of the manuscript; or in the review or approval of the manuscript. This work was supported by the by the Norwegian Health Directorate and the Research Council of Norway.
Acknowledgments: The REDIC-NH study was administrated by the Centre for Old Age Psychiatric Research, Innlandet Hospital Trust, and was initiated by the Norwegian Health Directorate, which also provided funding for the data collection. The first author’s Ph. D. study was funded by the Research Council of Norway.
Contributions of others who did not merit authorship but participated in the research: Nursing Homes in Hedmark, Oppland, Hordaland, and Nord-Trøndelag counties participated in the study. We would like to thank the residents and their next of kin for participating in the study and for giving us their information. We would also like to thank the nursing home managers for their cooperation, the staff members in the nursing home that filled out the questionnaires, and the research nurses that collected the data.
References
1. Wang SY, Shamliyan TA, Talley KM, Ramakrishnan R, Kane RL. Not just specific diseases: systematic review of the association of geriatric syndromes with hospitalization or nursing home admission. Archives of gerontology and geriatrics. 2013;57(1):16-26.
2. Moyle W, Fetherstonhaugh D, Greben M, Beattie E. Influencers on quality of life as reported by people living with dementia in long-term care: a descriptive exploratory approach. BMC geriatrics. 2015;15:50.
3. Lawton MP. Quality of life in Alzheimer disease. Alzheimer disease and associated disorders. 1994;8 Suppl 3:138-50.
4. Holopainen A, Siltanen H, Pohjanvuori A, Makisalo-Ropponen M, Okkonen E. Factors associated with the quality of life of people with dementia and with quality of life-improving interventions: Scoping review. Dementia (London, England). 2017:1471301217716725.
5. Klapwijk MS, Caljouw MA, Pieper MJ, van der Steen JT, Achterberg WP. Characteristics Associated with Quality of Life in Long-Term Care Residents with Dementia: A Cross-Sectional Study. Dementia and geriatric cognitive disorders. 2016;42(3-4):186-97.
6. Oudman E, Veurink B. Quality of life in nursing home residents with advanced dementia: a 2-year follow-up. Psychogeriatrics : the official journal of the Japanese Psychogeriatric Society. 2014;14(4):235-40.
7. van der Zon A, Wetzels RB, Bor H, Zuidema SU, Koopmans R, Gerritsen DL. Two-Year Course of Quality of Life in Nursing Home Residents with Dementia. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry. 2018.
8. Reimer MA, Slaughter S, Donaldson C, Currie G, Eliasziw M. Special care facility compared with traditional environments for dementia care: a longitudinal study of quality of life. Journal of the American Geriatrics Society. 2004;52(7):1085-92.
9. Anderson K, Bird M, MacPherson S, Blair A. How do staff influence the quality of long-term dementia care and the lives of residents? A systematic review of the evidence. International psychogeriatrics. 2016;28(8):1263-81.
10. Brooker D. What is person-centered care in dementia? Reviews in Clinical Gerontology. 2004;13(3):215-22.
11. Li J, Porock D. Resident outcomes of person-centered care in long-term care: a narrative review of interventional research. International journal of nursing studies. 2014;51(10):1395-415.
12. Testad I, Corbett A, Aarsland D, Lexow KO, Fossey J, Woods B, et al. The value of personalized psychosocial interventions to address behavioral and psychological symptoms in people with dementia living in care home settings: a systematic review. International psychogeriatrics. 2014;26(7):1083-98.
13. Chaudhury H, Cooke HA, Cowie H, Razaghi L. The Influence of the Physical Envirnment on Residents With Dementia in Long-Term Care Settings: A Review of the Empirical Literature. The Gerontologist. 2017;Vol. 00(00):1–13.
14. Roen I, Selbaek G, Kirkevold O, Engedal K, Testad I, Bergh S. Resourse Use and Disease Couse in dementia – Nursing Home (REDIC-NH), a longitudinal cohort study; design and patient characteristics at admission to Norwegian nursing homes. BMC health services research. 2017;17(1):365.
15. Roen I, Kirkevold O, Testad I, Selbaek G, Engedal K, Bergh S. Person-centered care in Norwegian nursing homes and its relation to organizational factors and staff characteristics: a cross-sectional survey. International psychogeriatrics. 2017:1-12.
16. Weiner M, Martin-Cook K, Svetlik D, Saine K, Foster B, Fontaine C. The quality of life in late-stage dementia (QUALID) scale 2000 [12818023]. 114-6].
17. Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. The British journal of psychiatry : the journal of mental science. 1982;140:566-72.
18. O’Bryant SE, Waring SC, Cullum CM, Hall J, Lacritz L, Massman PJ, et al. Staging dementia using Clinical Dementia Rating Scale Sum of Boxes scores: a Texas Alzheimer’s research consortium study. Archives of neurology. 2008;65(8):1091-5.
19. Husebo BS, Strand LI, Moe-Nilssen R, Husebo SB, Snow AL, Ljunggren AE. Mobilization-Observation-Behavior-Intensity-Dementia Pain Scale (MOBID): development and validation of a nurse-administered pain assessment tool for use in dementia. J Pain Symptom Manage. 2007;34(1):67-80.
20. Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. The Gerontologist. 1969;9(3):179-86.
21. Lyketsos CG, Galik E, Steele C, Steinberg M, Rosenblatt A, Warren A, et al. The General Medical Health Rating: a bedside global rating of medical comorbidity in patients with dementia. Journal of the American Geriatrics Society. 1999;47(4):487-91.
22. Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44(12):2308-14.
23. Selbaek G, Engedal K. Stability of the factor structure of the Neuropsychiatric Inventory in a 31-month follow-up study of a large sample of nursing-home patients with dementia. International psychogeriatrics. 2012;24(1):62-73.
24. Edvardsson D, Fetherstonhaugh D, Nay R, Gibson S. Development and initial testing of the Person-centered Care Assessment Tool (P-CAT). International psychogeriatrics. 2010;22(1):101-8.
25. Dallner M, Elo, A.-L., , Gamberale F, Hottinen V, Knardahl S, Lindström K. Validation of the general Nordic questionnaire (QPSNordic) for psychological and social factors at work. Copenhagen; 2000.
26. Sloane PD, Mitchell CM, Weisman G, Zimmerman S, Foley KM, Lynn M, et al. The Therapeutic Environment Screening Survey for Nursing Homes (TESS-NH): an observational instrument for assessing the physical environment of institutional settings for persons with dementia. The journals of gerontology Series B, Psychological sciences and social sciences. 2002;57(2):S69-78.
27. Mjorud M, Rosvik J, Rokstad AM, Kirkevold M, Engedal K. Variables associated with change in quality of life among persons with dementia in nursing homes: a 10 months follow-up study. PLoS One. 2014;9(12):e115248.
28. Naylor MD, Hirschman KB, Hanlon AL, Abbott KM, Bowles KH, Foust J, et al. Factors Associated With Changes in Perceived Quality of Life Among Elderly Recipients of Long-Term Services and Supports. Journal of the American Medical Directors Association. 2016;17(1):44-52.
29. Beerens HC, Zwakhalen SM, Verbeek H, Ruwaard D, Hamers JP. Factors associated with quality of life of people with dementia in long-term care facilities: a systematic review. International journal of nursing studies. 2013;50(9):1259-70.
30. van den Pol-Grevelink A, Jukema JS, Smits CH. Person-centred care and job satisfaction of caregivers in nursing homes: a systematic review of the impact of different forms of person-centred care on various dimensions of job satisfaction. International journal of geriatric psychiatry. 2012;27(3):219-29.