M.H. Itani1, G. Hamadeh2, S. Mroueh3, N. Naja4, O. Kolokotroni5
1. Department of Primary Care and Population Health, University of Nicosia Medical School, Cyprus; 2. Family Medicine Department, American University Hospital of Beirut, Lebanon; 3. Pediatric Department, American University Hospital of Beirut, Lebanon; 4. Dar Al-Ajaza Al-Islamia Hospital, Geriatric Department, Beirut-Lebanon; 5. Department of Primary Care and Population Health, University of Nicosia Medical School, Cyprus. Corresponding author: 1. Mohamad H. Itani MSc, Department of Primary Care and Population Health, University of Nicosia Medical School, Cyprus, mhitani57@gmail.com
Jour Nursing Home Res 2020;6:61-68
Published onlineSeptember 1, 2019, http://dx.doi.org/10.14283/jnhrs.2020.18
Abstract
Objectives: Sleep problems are common in institutionalized elders and are associated with increased mortality and morbidity. Our study evaluates the prevalence of poor sleep quality in a chronic care philanthropic institution and their possible predictors in that setting. Methods: The study involved 192 participants. The Pittsburgh Sleep Quality Index (PSQI) scale was used as an assessment tool for poor sleep quality. Participants with a global PSQI score of 5 or more were classified as poor sleepers. Bivariate analysis was used to study statistical correlation between high PSQI scores and the independent variables of demographic data and lifestyle habits, coexisting chronic medical conditions and current medications. Results: Poor sleep quality was present in 75% of the participants. Decreased ambulatory activity, poor performance on memory tests and Alzheimer disease had positive association with poor sleep quality [OR 2.85 (95% CI, 1.41-5.68), OR 4.21 (95% CI, 1.90-9.33) and OR 2.28 (95% CI, 1.05-4.94) respectively]. Schizophrenia had a negative association [OR 0.46 (95% CI, 0.23-0.92)], while proton pump inhibitors approached statistical significance [OR 1.85 (95% CI, 0.96-3.59)]. Conclusion: Our study showed high prevalence of poor sleep quality in institutionalized elders. Physicians must use a standardized tool for assessment of sleep in that setting and not rely on a single question about sleep quality. The activity of moving the elders out of the bed during the day is a simple intervention that may help in improving their sleep at night. The association between poor sleep and Alzheimer disease and impaired memory reflects the importance of early identification of these problems and early management. Overuse of proton pump inhibitors in institutionalized elders may be associated with poor sleep quality.
Key words: Institutionalized elders, sleep quality, Alzheimer disease, Pittsburgh sleep quality index.
Introduction
Elderly people have increased prevalence of sleep problems secondary to physiological decline in their circadian sleep rhythm, coexisting medical diseases and medications (1-3). In an epidemiological study on independently living seniors followed-up for 3 years, 50% of the survivors who had chronic insomnia reported improvement in their health after control of their sleep problems (2). This reflects the importance of sleep control on the general wellbeing of seniors. Elderly people living in chronic care institutions represent a distinct population characterized by frailty, cognitive impairment decreased physical activity and increased prevalence of specific sleep disorders compared to community dwelling population (4). On the other hand, disturbed sleep in elders living in chronic care centers was found to be associated with increased incidence of falls and mortality (5, 6).
There is paucity of reports in the medical literature on the sleep problems in institutionalized elders living in low and middle-income countries (7). The purpose of this study is to investigate the prevalence of poor sleep quality in a cohort of institutionalized elders in a high middle-income country and to identify their possible predictors.
Methods
Study Design and Population
This is a cross-sectional study conducted from December 11, 2017 through February 10, 2018 in a philanthropic long stay institution for elders located in Beirut – Lebanon. Residents are assigned 4-8 to a room for sleeping. They have a common hall where they watch television and rest and share common bathrooms.
Our study got the approval of the institutional Scientific Research and Ethics Committee in November 15, 2017. It was conducted in accordance with the national and international ethical guidelines for human research on geriatric patients (8, 9).
Participants were divided into two groups according to their cognitive function, based on the results of their mini mental state examination (MMSE) test, the clock-drawing test or both that were performed within 1 week prior to the study. Participants with a score of 25 and above on MMSE test were considered to pass the test while those with lower scores were considered to fail the test. For participants who underwent clock drawing test, participants with a score of 2 points were considered to pass the test and those with a score of less than 2 points were considered to fail the test. Participants with previous test results underwent clock-drawing test evaluation prior to their enrollment. Those who passed either MMSE test or clock-drawing test or both were deemed cognitively competent and signed an informed consent form (ICF) for participation. Those who failed, had the ICF signed by their legal representative if available or otherwise by the hospital administration which was legally their guardian during their hospital stay.
Inclusion criteria were to be a resident of the institution for at least 1 month prior to the start of the study and age of sixty years or above. Exclusion criteria were: diagnosis of a sleep disorder (like sleep apnea, hypopnea, insomnia, parasomnia, hypersomnia, restless leg syndrome and sleepwalking), acute psychiatric illness, medical conditions that could lead to severe cognitive deterioration including renal, respiratory, cardiac, and hepatic diseases, a stroke or traumatic head injury within one month or any recent acute illness or trauma within 2 weeks prior to the start of the study.
Measurements
We used the Pittsburgh Sleep Quality Index (PSQI) scale which is a standardized tool for assessment of quality and patterns of sleep in older adults (Appendix-I) (10). It differentiates between “poor sleep quality” and “good sleep quality” by measuring seven sleep components. It has an internal Cronbach’s alpha consistency and reliability coefficient of 0.70-0.83 in different studies for its seven components (11). Each component is assigned a score from 0 to 3 with a maximum global score of 21. Participants with a global PSQI score of 5 or more are classified as “poor sleepers”. The questionnaire relies on self-reporting except for question 5-E (Appendix-I) which says “During the past month how often have you had troubles sleeping because you cough or snore loudly?” which should be answered by a person who knows the participant, in our setting the nurse taking daily care of the participant.
The PSQI scale is a self-reporting tool. However, since a good number of our participants were cognitively non-competent to fill the questionnaire on their own, we asked the nurse who was taking daily care of the cognitively non-competent participant and knew him/her best to fill the PSQI questionnaires on their patients’ behalf. To have a standardized response in this process from the nurses, the primary investigator met with the nurses before filling the questionnaire. He explained to them the objective of the study and reviewed with them the items of the PSQI scale, item by item, to make sure they understand the objectives of each question and its scoring mechanism. Some questions were very subjective and challenging in term of nurses’ response. For example, question 5-H which says, “During the past month, how often have you had trouble sleeping because you have bad dreams?”. To standardize the nurses’ response to that question, nurses were asked to report the frequency of awakening at night associated with horror or fear as an indicator of bad dreams and to score its presence or absence accordingly. Similarly questions 5-F and 5-G which says, “During the past month, how often have you had trouble sleeping because you feel too hot or too cold?” The nurses were asked to respond to that question by checking on the number of blankets/covers used by the participant at night. Use of extra blankets associated with troubled sleeping was considered to reflect feeling too cold and removal of blankets with troubled sleeping reflected feeling too hot.
Finally, question 9 of the PSQI scale which says, “During the past month, how would you rate your sleep quality overall?” was used as a single question for subjective assessment of participant’s sleep quality. Participants who reported “fairly bad” or “very bad” sleep quality were considered to have subjective feeling of bad or poor sleep quality in our analysis. As for the non-competent participants, it was the nurse caregiver who made the decision about the quality of sleep of their patients based on their experience in that respect.
The total number of hours spent in bed during the day, retrieved from PSQI question 4-B, was used as a measure of the participant’s ambulatory physical activity during the day. In our analysis, we considered participants who stayed in bed more than 12 hours per day to have decreased ambulatory activity and compared their global PSQI scores to those of the other participants.
The English version of the PSQI scale was translated into Arabic following the WHO guidelines (12) by a professional health translator. The Arabic version was back translated into English to generate a new English version that was compared with the original version by an English language expert who confirmed conceptual similarity. The revised Arabic version and the original English version were then pre-tested on a sample of twelve bilingual persons aged between 65 and 70 years. The responses to each question in either version were compared and in case of discrepancy, the question was modified to get a consistency of 100%. The final Arabic PSQI version was used in our study.
Statistical Analysis
Descriptive statistics were presented as counts and percentages for categorical variables, and mean and standard deviation, or median and interquartile range for quantitative variables. The dependent variable was the global PSQI score which was dichotomized into a negative score with a global score less than 5, and a positive score equal to or above 5. The latter score was considered to reflect the presence of sleep problem. Our independent variables included demographic data and lifestyle, in addition to coexisting chronic medical conditions and current medications.
The PSQI score (our dependent variable) was tested for statistical association with the independent variables on bivariate analysis using Chi-square tests to compare percentages, or Fisher’s exact test in case of low cell counts, and t-test to compare means. Results were presented as odds ratio with 95% confidence interval using simple logistic binary regression.
All variables which were significant or close to significance on bivariate analysis were included later in a stepwise logistic regression to determine the best model associated with presence of poor sleep quality. Results of the multivariate analysis were presented in the final model as adjusted odds ratios with 95% confidence intervals. The level of significance alpha was fixed at P-value of 0.05. Statistical analysis was run by using IBM SPSS statistical software.
Results
A total of 247 seniors were living in the institution at the time of the study. 192 residents (98 males and 94 females) fulfilled our inclusion criteria and participated in the study. Fifty-five residents were excluded: 52 residents because they were younger than sixty and three because of acute illnesses during the previous two weeks. Participants were 60 to 102 years old with a mean age of 77.4.
Compared to male participants, female participants were older. They were more likely to be overweight or obese, unemployed prior to admission to the institution, not to smoke, to stay more than 12 hours in bed, to report sleep problems and to perform poorly on memory tests.
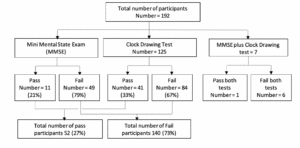
Figure 1 summarizes the participants’ performance on memory tests. A total of 52/192 (27%) residents passed the memory tests: 41 (79%) the clock drawing test, 11 (21%) the MMSE test, and one both. Six participants failed both tests.
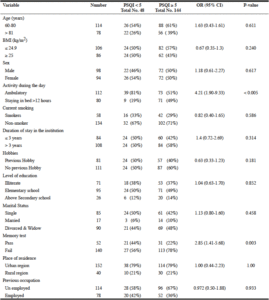
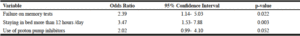
Table 1, summarizes the results of the statistical analysis of the selected demographic and lifestyle variables and their corresponding global PSQI scores. One hundred-forty-four participants had a global PSQI scores ≥ 5, making the prevalence of poor sleep quality in our population approximately 75% (61% and 81% among competent and non-competent participants respectively). The prevalence of subjectively perceived bad sleep quality as assessed by question 9, was 27.6 % (28% and 27% among competent and non-competent participants respectively). Of the remaining demographic and lifestyle variables, failure on memory tests and staying in bed for more than 12 hours during the day were the only variables positively associated with poor sleep quality (OR 2.85, 95% CI 1.41-5.68 and OR 4.21, 95% CI 1.90-9.33 respectively).
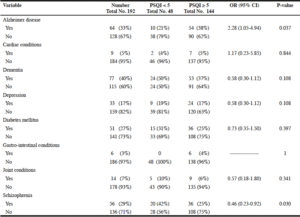
Table 2, summarizes the results of the statistical analysis of the chronic medical conditions and their corresponding global PSQI scores. Only Alzheimer disease was positively associated with poor sleep (OR 2.28, 95% CI 1.05-4.94), while schizophrenia was associated with good sleep quality (OR 0.46, 95% CI 0.23-0.92).
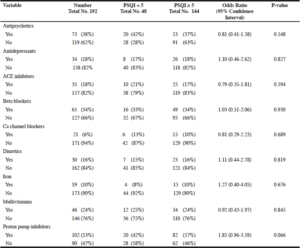
Table 3 summarizes the results of the statistical analysis of the medications taken by the participants during the study period and their corresponding global PSQI scores. Of the listed medications proton pump inhibitors (PPI) were the only medicines that were close to a statistical association with poor sleep quality (OR 1.85, CI 0.96-3.59).
The final best-chosen model in our stepwise logistic regression was failure on memory tests, staying for long time in bed and intake of proton pump inhibitors (Table-4). The p-value for that model was <0.0001. The three independent variables could explain together around 10.7 % of the variability in poor sleep quality.
Discussion
Our study showed poor sleep quality to be prevalent in 75% of our population by using the PSQI global scores, while only 27.6% reported poor sleep quality when asked a single direct question. Although the PSQI is a self-reporting scale, our strategy of asking the nurses taking direct care of cognitively non-competent participants to report the PSQI questionnaires on behalf of their patients may be associated with reporter bias. In spite of that, our results show that in both the competent self-reporting group and the non-competent nurses reporting group, a high prevalence of poor sleep quality was revealed by using PSQI global score (61% and 81% respectively), compared to the single question about sleep quality where the bias was less evident (28% and 27% respectively). This finding is consistent with findings by Mansano et al. (7) who studied 37 elderly persons living in a long-term care facility in Brazil. They found a prevalence of sleep problems in 81% of their participants, using a special sleep evaluation questionnaire, while the self-reported prevalence was 30%. Their study and ours show that a single question inquiry about sleep quality in elder people results in underestimation of the actual burden of the problem compared to a standardized questionnaires like those of the PSQI scale, which consists of several questions addressing different aspects of the sleeping behavior.
Our study found an association between poor sleep quality and decreased ambulatory activity of elders during the day, defined in our study as staying in bed daily for more than 12 hours. The relationship between physical activity and sleep problems is poorly understood (4). Morais et al. found that moderate acute aerobic exercise for 30 minutes twice weekly improves cognitive function, depressive symptoms, fatigue severity and functional capacity in patients with chronic stroke (13). That improvement was associated with increased levels of brain-derived neurotrophic factor, which has been shown to be critical in the process of homeostatic regulation of rapid eye movement sleep (14). Our participants did not participate in any physical activity program, yet those who left their beds during the day, moved around within the pavilion premises had better sleep quality than those who stayed in bed for longer periods. On the other hand, Valentine et al. found a significant association between sleep quality, adiposity and physical activity in older adults (15). We however did not find a statistically significant association between poor sleep quality and participants with BMI > 25 kg/m2. This is probably due to the small number of obese participants in our study who were grouped and analyzed with the overweight participants (Table 1).
Coexisting chronic medical conditions in elders have been previously identified to be associated with sleep problems (1). A chronic medical condition is broadly defined as any condition that lasts one year or more and requires ongoing medical attention or limits activities of daily living or both. Heart disease, diabetes mellitus, or cancer are among the leading chronic diseases in that respect. Uncontrolled diabetes mellitus and heart failure, poorly controlled depression and generalized anxiety are known causes of sleep disordered breathing (22, 23). All our study participants had their chronic medical conditions controlled. In addition there were only few cases that had chronic heart disease (9 cases) and gastrointestinal disease (6 cases), to be considered in any statistical analysis.
Dementia and Alzheimer disease (AD) were the most prevalent chronic medical conditions in our population. Only AD was positively associated with poor sleep quality. Dementia is defined as an acquired brain syndrome characterized by a decline in cognitive functioning not entirely attributable to normal aging, and significantly interfering with independence in the person’s performance of activities of daily living. The impairment involves two or more cognitive domains as memory, executive functions, attention, language, social cognition and judgement, psychomotor speed, visuoperceptual or visuospatial abilities (16).
Alzheimer disease on the other hand, is an advanced and irreversible neurocognitive condition, characterized by progressive loss of memory and thinking skills and, eventually, the ability to carry out the simplest tasks. Clinically it is characterized by progressive dementia, debilitation, cognitive and functional impairment and behavioral challenges (17). Pathophysiologically, AD is associated with deposition of β-amyloid plaques and tau proteins in the brain of affected patients. Our finding about the association between AD and poor sleep is consistent with the literature (18, 19). Preexisting poor sleep contributes to the pathogenesis of AD through two mechanisms that increase β-amyloid deposition in the brain: promotion of β-amyloid peptide production by increasing neuronal firing during sleep loss and impairment of clearance of the β-amyloid brain waste, which occurs during the slow-wave sleep (18).
Our study also showed an association between poor memory but not dementia and poor sleep quality. This could be another effect of increased amyloid burden. Hedden et al. found an association between amyloid burden and memory loss in a subset of cognitively normal older adults. They suggest that such effect is small, yet it likely represents the earliest stages of neuropathology that progresses toward clinically detectable memory impairment later on (20).
Schizophrenic participants in our study tended to have better sleep quality with lower PSQI global scores. Joober et al. in a literature review about insomnia in schizophrenic patients suggested an algorithm to improve sleep in that population (21). Our schizophrenia participants were medicated according to that algorithm, which may explain the positive association between their disease and sleep quality.
Most of our participants were on multiple medications, which increases the risks of drug interactions and adverse reactions. Full discussion of drugs and sleep problems is beyond the scope of this paper and readers are referred to a chapter written by Malangu (24). In our study, only intake of PPI reached close statistical association with poor sleep quality (OR 1.85, CI 0.96-3.59). However, our best-chosen model of poor memory, staying in bed for more than 12 hours during the day and intake of PPI (Table 4), had a 10.7% predictive value regarding poor sleep quality with a p-value of <0.0001 on stepwise logistic multivariate regression, which suggests that PPI increases the risk of poor sleep quality when considered within that model. This possible association could have resulted from overuse of PPI in our population, PPI were used in 102 participants, while only 6 participants had documented gastrointestinal diseases (Table-2). The use of PPI as part of a polypharmacy in elders increases the risks of drug interactions and potential unexpected side effects like poor sleep. Another explanation for this finding could be that PPI had been prescribed for elders with sleep problems to treat gastroesophageal reflux as a presumed cause of their poor sleep. These two possibilities call for caution in PPI prescription for elders. In any case, this finding needs further evaluation in future studies.
Our study evaluated the impact of socio-demographic and lifestyle factors like age, sex, education, social status, hobbies, employment versus non-employment, rural versus urban background, smoking habits, ambulatory daily activity and performance on memory tests, in addition to coexisting chronic medical conditions and medications on the quality of sleep in institutionalized elders. Out of all the studied variables decreased ambulatory activity and poor performance on memory tests were the only variables associated with poor sleep quality. There may have been other environmental and operational variables that were not considered in our study design. Gordon et al (4) in a literature review of randomized controlled trials conducted in care home setting, found that sleep disorders were attributed to accumulation of general medical conditions associated with insomnia like heart failure, Parkinson disease, dementia, depression and restless leg syndrome most of which were excluded in our study design. In their conclusion, they stressed on the importance of few environmental and operational variables like bright light exposure during the day, physical activity, frequency of nighttime checks, nighttime light and noise level. In our setting, the participants lived in a pavilion with poor exposure to bright light during the day, in crowded rooms with limited number of toilets and no control on the resulting noise. Operationally, they had limited number of nursing staff with lack of programmed routine naptime and lack of physical activity and frequent nighttime checks. These factors were not adequately addressed in our study and may have affected our participants’ sleep quality results.
Conclusion
Our study sheds light on the high prevalence of poor sleep quality in institutionalized elders living in a philanthropic center in a high middle-income country. It shows that physicians must use a standardized sleep assessment tool and not rely on a single question response in their evaluation of sleep quality in this population. It suggests the use of proxy nurse reporter as alternative for self-reporting of PSQI scale in cognitively non-competent elders. It shows the importance of ambulatory daily activity in improving sleep quality in seniors. It brings the issue of PPI overprescription and its possible association with poor sleep and calls for revision of their indications in elders. The association between sleep problems, β-amyloid deposition in brain and memory loss suggests that further studies need to be undertaken to address the effects of healthy sleep habits on memory in elders.
Conflict of interest: The authors declare that they have no conflict of interest and that they did not receive any funding from any pharmaceutical company or other funding agency to accomplish this study. They have no affiliations with or involvement in any organization with any financial or non-financial interest in the subject matter or materials discussed in this manuscript.
Acknowledgement: We thank each of the doctors Mahmoud Faour, Faysal Chatila, Nadia Ramadan and the nursing staff at Dar Al-Ajaza Al-Islamia for their great logistic and technical support and Dr Sani Hlais for statistical analysis.
Ethical standards: Ethical approval for the study was obtained from Dar Al-Ajaza Al-Islamia Scientific Research and Ethics Committee.
References
1. Foley, D. J., Monjan, A., Simonsick, E. M., Wallace, R. B., & Blazer, D. G. Incidence and remission of insomnia among elderly adults: An epidemiologic study of 6,800 persons over three years. Sleep, 1999;22 Suppl 2, S366-72.
2. Foley, D. J., Monjan, A. A., Brown, S. L., Simonsick, E. M., Wallace, R. B., & Blazer, D. G. Sleep complaints among elderly persons: An epidemiologic study of three communities. Sleep, 1995;18(6), 425-432.
3. Vitiello, M. V., Moe, K. E., & Prinz, P. N. Sleep complaints cosegregate with illness in older adults: Clinical research informed by and informing epidemiological studies of sleep. Journal of Psychosomatic Research, 2002;53(1), 555-559.
4. Gordon, A, L., & Gladman, J. R. Sleep in care homes. Review in Clinical Gerentology, 20, 309-316. Doi: 10.1017/S0959259810000250
5. Brassington, GS., King, AC., & Bliwise, DL. Sleep problems as a risk factor for falls in a sample of community-dwelling adults aged 64-99 years. J Am Geriatr Soc, 2000;48, 1234-40. doi:10.1111/j.1532-5415.2000.tb02596.x.
6. Dale, MC., Burns, A., Panter L., & Morris, J. Factors affecting survival of elderly nursing home resident. Int J Geriatr Psychiat, 2001;16, 70-76.
7. Mansano-Schlosser, T. C., dos Santos, A. A., Camargo-Rossignolo Sde, O., Freitas, D. C., Lorenz, V. R., & Ceolim, M. F. Institutionalized elderly: Chronological organization of daily routines and sleep quality. [Idosos institucionalizados: organizacao cronologica das rotinas diarias e qualidade do sono] Revista Brasileira De Enfermagem, 2014;67(4), 610-616.
8. Ilgili, O., Arda, B., & Munir, K. ( Ethics in geriatric medicine research. Turk Geriatri Dergisi, 2014;17(2), 188-195.
9. Fields, L. M., & Calvert, J. D. ( Informed consent procedures with cognitively impaired patients: A review of ethics and best practices. Psychiatry and Clinical Neurosciences, 2015;69(8), 462-471.
10. Buysse, D. J., Reynolds, C. F.,3rd, Monk, T. H., Berman, S. R., & Kupfer, D. J. The pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Research, 1989;28(2), 193-213.
11. Mollayeva, T., Thurairajah, P., Burton, K., Mollayeva, S., Shapiro, C. M., & Colantonio, A. The pittsburgh sleep quality index as a screening tool for sleep dysfunction in clinical and non-clinical samples: A systematic review and meta-analysis. Sleep Medicine Reviews, 2016;25, 52-73.
12. World health organization programmes: Process of translation and adaptation of instruments. http://www.who.int/substance_abuse/research_tools/translation/en/
13. Morais, VAC., Tourino, MFDS., Almeidam, ACS., Albuquerque, TBD., Linhares, RC., Christo, PP., Martinelli, PM., & Scalzo, PL. A single session of moderate intensity walking increases brain-derived neurotrophic factor (BDNF) in the chronic post-stroke patients. Top Stroke Rehabil, 2018;25(1), 1-5. doi: 10.1080/10749357.2017.
14. Datta, S., Knapp, C. M., Koul-Tiwari, R., & Barnes, A. The homeostatic regulation of REM sleep: A role for localized expression of brain-derived neurotrophic factor in the brainstem. Behavioural brain research, 2015;292, 381–392. doi:10.1016/j.bbr.2015.06.038
15. Valentine, R. J., Woods, J. A., McAuley, E., Dantzer, R., & Evans, E. M. The associations of adiposity, physical activity and inflammation with fatigue in older adults. Brain, Behavior, and Immunity, 2011; 25(7), 1482-1490.
16. National institute on aging. Basics of Alzheimer’s disease and dementia. What is alzheimer’s disease? https://www.nia.nih.gov/health/what-alzheimers-disease
17. International statistical classification of diseases and related health problems 10th Revision – WHO. Dementia (F00-F03). https://icd.who.int/browse10/2016/en#/F01
18. Malhotra RK. Neurodegenerative disorders and sleep. Sleep Mned Cli, 2018;13(1), 63-70.
19. Spira, A. P., & Gottesman, R. F. Sleep disturbance: An emerging opportunity for alzheimer’s disease prevention? International Psychogeriatrics, 2017;29(4), 529-531.
20. Hedden, T., Mormino, E. C., Amariglio, R. E., Younger, A. P., Schultz, A. P., Becker, J. A., et al. Cognitive profile of amyloid burden and white matter hyperintensities in cognitively normal older adults. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 2012;32(46), 16233-16242.
21. Joober, R., Cole, K., Tabbane, K., & Boivin, D. B. An algorithmic approach to the management of insomnia in patients with schizophrenia. Annals of Clinical Psychiatry : Official Journal of the American Academy of Clinical Psychiatrists, 2017;29(2), 133-144.
22. Nakata, K., Miki, T., Tanno, M., Ohnishi, H., Yano, T., Muranaka, A., et al. Distinct impacts of sleep-disordered breathing on glycemic variability in patients with and without diabetes mellitus. PloS One, 2017;12(12), e0188689.
23. Roepke, S. K., & Ancoli-Israel, S. Sleep disorders in the elderly. The Indian Journal of Medical Research, 2010;131, 302-310.
24. Malangu, N. Drugs inducing insomnia as an adverse effect. In S. Sahoo (Ed.), Can’t sleep? issues of being an insomnia, 2012;pp. 23-26, Intechopen.