J. Wearing1,2, M. Stokes3,4, R.A. de Bie1, E.D. de Bruin5,6
1. Department of Epidemiology, Faculty of Health, Medicine and Life Sciences, School for Public Health and Primary Care, Maastricht University, Maastricht, The Netherlands; 2. Adullam Spital und Pflegezentren, Basel, Switzerland ; 3. School of Health Sciences, University of Southampton, Southampton, United Kingdom; 4. Centre for Sport, Exercise and Osteoarthritis Research Versus Arthritis, Nottingham, United Kingdom; 5. Institute of Human Movement Sciences and Sport (IBWS) ETH, Department of Health Sciences and Technology, ETH Zurich, Zürich, Switzerland; 6. Division of Physiotherapy, Department of Neurobiology, Care Sciences and Society, Karolinska Institutet, Stockholm, Sweden. Corresponding author: Julia Wearing, Department of Epidemiology, Faculty of Health, Medicine and Life Sciences, School for Public Health and Primary Care, Maastricht University, Maastricht, The Netherlands; E-mail: j.wearing@bluewin.ch, Phone: +41 61 2669799
Jour Nursing Home Res 2020;6:93-99
Published online October 22, 2020, http://dx.doi.org/10.14283/jnhrs.2020.25
Abstract
Background: Handgrip strength and a chair-stand-test are often used to evaluate strength and function, and to detect probable sarcopenia in community-living, older adults. In institutionalized, frail older people, evaluation of muscle performance is of particular importance but it has received little attention. Objectives: To evaluate the feasibility of handgrip strength and the chair stand test in nursing-home residents, and their relation to overall strength, daily functioning and frailty. Design: A cross-sectional study. Setting: A nursing-home in Switzerland. Participants: 30 nursing-home residents, 23 women, age median (range) 86.5 (68-103) years. Measurements: Handgrip strength, the chair stand test, knee extensor and elbow flexor strength, gait speed, activities of daily living and frailty were assessed. The Mann-Whitney-U Test was used to compare sub-groups of sarcopenia (probable sarcopenia versus no probable sarcopenia) while Cohen’s Kappa and Area under the Receiver Operating Characteristic curve examined relationships between tests. Results: All participants were able to perform the handgrip strength test, while only 14 could complete the chair rise test. Probable sarcopenia was detected by handgrip strength in 22 and chair stand test in 24 (8 slow; 16 unable to complete) participants, with an overlap of 19. Probable sarcopenia, detected by each of the tests, was significantly associated with low gait speed and severe frailty status, while low handgrip strength also indicated low elbow flexor and knee extensor strength, and high dependence in activities of daily living. Conclusions: Handgrip strength test is superior to the chair stand test as a strength test to detect probable sarcopenia in nursing-home residents, as it could be completed by more frail people. Sarcopenia-specific cut off values in handgrip strength indicated overall strength, leg function, performance of daily activities and frailty, hence, the test could be used as a screening test for physical condition. Although further research is needed, given the importance of detecting muscle performance, handgrip strength testing is recommended in nursing-home residents.
Key words: Probable sarcopenia, nursing-home residents, handgrip strength, chair stand test, frailty.
Introduction
Muscle strength is a very important prerequisite for healthy aging (1). It is a predictor of adverse health outcomes, such as dependence in activities of daily living (ADL) and mortality (2) in community-living older adults. Older people whose strength drops significantly due to chronic diseases or inactivity, and who lose independence in daily activities, receive support from home care or become institutionalised in long-term care (3). At admission to the institution, people are assessed for their need of care. However, standardized assessment of muscle status is typically not undertaken (4) even though nursing-home residents are at risk for further strength and functional decline (3, 5). Rather, residents are specifically evaluated for physical capacity only when negative consequences of functional decline, such as falls, occur. Since low muscle strength and function can be improved even in frail older people (6), strength testing is considered important in order to prescribe tailored interventions in a timely manner.
The two strength tests, handgrip strength (HGS) and chair stand test (CST), are quick and easy to perform and reportedly meaningful for health-related outcomes in community-dwelling older people (7). The European Working Group for Sarcopenia in Older People (EWGSOP2) advocates these two tests with distinct cut-off values to detect those likely to have sarcopenia (probable sarcopenia): the HGS of <16 kg for women and < 27 kg for men, and a CST of > 15 seconds (8). In case of probable sarcopenia detection, recommended interventions are initiated to improve strength even if the diagnosis of sarcopenia is not/not yet confirmed (8), as prevention of decline is vital. However, strength testing in long-term care has been little explored (5), despite the urgent need for specific information about muscle performance in this population. Feasibility of strength tests in older nursing-home residents to detect probable sarcopenia is questionable (7, 9). Moreover, the indicative value of probable sarcopenia detected by the EWGSOP2 guidelines for health-related outcomes, such as frailty and ADL dependence, in older nursing-home residents has not been explored to date. The objectives of this study, therefore, were to:
1. Evaluate the feasibility of HGS and CST in nursing-home residents
2. Evaluate the prevalence of probable sarcopenia detected by each test, according to cut off values defined by the EWGSOP2
3. Explore the differences between the sub-groups with/without probable sarcopenia in regard to strength, function, ADL dependence, comorbidities and frailty
Methods
Study design
An observational, cross-sectional study to assess muscle strength, physical function and frailty was undertaken in Swiss nursing-home residents between August and December 2017.
Participants
Older adults, aged 65 years and over, were screened for exclusion criteria by a certified nurse based on the RAI (Resident Assessment Instrument) which is routinely performed in nursing-home residents. Exclusion criteria were: a) severely impaired decision making (Cognitive performance scale > 4 points); b) a history of acute lower limb pathology (fracture and/or surgery within the last 6 months); c) limb paralysis; and d) confinement to bed. Volunteers who were able to understand study content and signed informed consent, were included in the study. All study procedures complied with the principles of the Declaration of Helsinki for ethical research in humans and the study received approval from the local ethics committee (project-ID 2017-00839).
Sample size
Sample size (n=30) was estimated a priori based on previously published data on prevalence of sarcopenia in nursing-home residents (10). The calculated number would be sufficient to detect a prevalence of 50% at a 90% confidence level and with 85% precision (d = 0.15).
Data collection
Strength measures, the CST, gait speed and the frailty assessment were examined by a physiotherapist trained and experienced in musculoskeletal assessments.
HGS was measured with a hydraulic hand dynamometer (Jamar®, Lafayette, USA) according to the standardized protocol of the American Society of Hand Therapists (11). The maximal value of two trials was used to identify residents with and without probable sarcopenia according to a cut-off value of 16 kg for women and 27 kg for men, as defined by the EWGSOP2 (8).
CST was performed according to a standardized protocol, published by Guralnik (12). The test involves the completion of five chair rises from a full-seated position to upright stance in as short time as possible with the arms crossed over the chest. The time for completion of five chair stands was measured with a stop watch. Classification of “probable sarcopenia” or “no probable sarcopenia” was based on a cut off value of 15 seconds (8).
Participant demographics, medical history, cognitive performance and self-performance in ADL were obtained using the RAI (Minimum Data Set Version 2.0). For evaluation of cognition and ADL, participants’ performance was closely observed by trained nurses and then encoded with the standardized RAI-item coding system.
a) Age was reported in years, height in meters and weight in kg.
b) Medical history included chronic diseases of the metabolic, musculoskeletal, neurological, and respiratory system, psychiatric conditions, renal insufficiency, vertigo and cancer. Number and type of diseases were recorded.
c) Cognitive performance was classified on the Minimum Data Set Cognitive Performance Scale ranging from 0 (= intact cognition) to 6 (= severely limited cognition) points (13).
d) Basic ADL included 10 usual daily activities of nursing-home residents: bed mobility, transfer, dressing, eating/drinking, toilet use, personal hygiene, walking in a room and in a corridor, locomotion on and outside the ward. Each of the 10 activities was rated on a scale from 0 (independent) to 4 (fully dependent), with a full range of 0–40, based on the performance of the last 7 days. Participants were categorized as a) independent in ADL when total score was 0, and as dependent in ADL when total score was ≥ 1, which reflected assistance or staff oversight in at least one activity.
Maximum voluntary isometric contraction strength of the knee extensor and elbow flexor muscles was measured using a hand-held dynamometer (Microfet2®, CompuFET, Hoggan Health Industries, Biometrics Europe). For measurement, the participant was seated with their back resting against a firm support, thighs fully supported. Knee and elbow were flexed at 90° respectively while the participants were asked to push against the dynamometer as hard as possible. The highest value of two trials was recorded. Intraclass Correlation Coefficient (ICC) reported for repeated measures of hand-held dynamometry range between 0.90 and 0.98 in older adults (14).
Habitual gait speed (m/s) was evaluated over a 4-meter, level walkway at participant’s preferred speed. Time was recorded to the nearest hundredth of a second with a stopwatch. Participants were permitted the use of a walking aid. Test-retest reliability of gait speed assessments recorded over comparable distances have been shown to be adequate (ICC of 0.715) and related to measures of physical function (r = 0.554) in older individuals (15).
Physical frailty was evaluated according to Fried’s frailty criteria (16), namely unintentional weight loss < 5kg in the past year, weakness (low HGS), exhaustion (self-report), slowness (slow walking speed) and low physical activity. Participants were classified as “pre-frail” in case of 1-2 positive criteria and as “frail” in case of 3-5 positive criteria.
Statistical Analysis
IBM SPSS Statistics, Version 23 was used for statistical analysis. The Mann-Whitney-U Test was used to compare the sub-groups, probable sarcopenia versus no probable sarcopenia, with respect to strength, physical function, ADL performance and frailty. Feasibility was based on the number of people that were able to complete the EWGSOP2 advocated screening tests for detection of probable sarcopenia. Cohen’s Kappa and the area under the Receiver Operating Characteristic (ROC) curves (AUC) were applied for relationships between the two detection tests and accuracy of the tests to detect frailty status and gait speed.
Results
Descriptive characteristics Of a total of 30 nursing-home residents with median (range) age 86.5 (68-103) years, height 1.62 (1.49-1.72) m and weight 66.5 (35-95) kg, 23 were female. Prefrailty was detected in 13, frailty in 17 participants, and 29 had more than two chronic diseases. The cognitive performance, with 0 being cognitively intact, was median (range) 1 (0-3).
Feasibility of test performance All participants could perform the HGS test, however only 14 participants (47%) could successfully get up from a chair at all. Subsequently, participants who could not complete the CST (n=16) and those who performed slower than the cut off value (n=8) were combined as the slow/no CST group.
Prevalence of probable sarcopenia Low HGS was prevalent in 78% (n=22) while the prevalence of probable sarcopenia detected by slow/no CST was 80% (n=24). Cohen’s Kappa showed an overlap of n=19 between the people assessed by low HGS and those assessed by slow/no CST. The Kappa value of 0.259 (95%CI 0.293-0.311) demonstrates a fair relationship between the two tests (17) (Table 1).
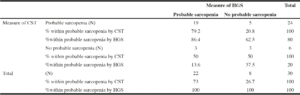
Table 1
Crosstabulation for prevalence and overlap of people with probable sarcopenia detected by low HGS and slow/no CST
Differences between the sub-groups with/without probable sarcopenia Participants with probable sarcopenia detected by low HGS also had lower elbow flexor and knee extensor strength, slower gait speed, were more often dependent in ADL and had more symptoms of frailty than people without (p < .05). However, there was no significant difference in age, height, weight, comorbidities or the ability to perform the CST between sub-groups (Table 2).
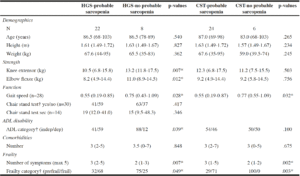
Table 2
Differences (median (min-max)) between participants with and without probable sarcopenia detected by low HGS
and low/no CS
Continuous variables are documented as median (range); *p-values with exact significance, 2-tailed; †categorical variables are presented as percentages
Participants with probable sarcopenia detected by slow CST, had significantly slower gait speed and more frailty symptoms than those without. Age, height, weight, strength, ADL performance and comorbidities did not differ between sub-groups (Table 2).
The AUC showed that probable sarcopenia detected by HGS distinguished between frailty statuses to 72% and gait speed to 77%. The CST distinguished between frailty statuses to 85% and gait speed to 79% (Fig. 1 and 2).
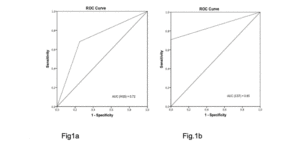
Figure 1
ROC curve and AUC: handgrip strength (1a) and chair stand test (1b) accuracy in discriminating frailty status
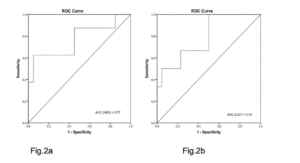
Figure 2
ROC curve and AUC: handgrip strength (Fig.2a) and chair stand test (Fig.2b) accuracy in determining gait speed
Discussion
This group of 30 nursing-home residents was heterogeneous in their health and frailty status. However, the participants are representative of institutionalized, older people in regard to muscle strength and physical function (18).
The feasibility of performing the two EWGSOP2 advocated tests differed significantly in this group of nursing-home residents. While all participants could perform the HGS test, more than half of the residents could not stand up from a seated position without the use of their upper limbs. Oldest-old nursing-home residents often experience an excessive loss in muscle strength which might drop below the necessary threshold needed for standing up (19). Hence, the floor effect of the CST observed in this study might limit its usefulness in this population even if participants who have not been able to perform the test were also classified as having probable sarcopenia, as were participants who had results below the threshold. Modifications of the proposed CST performance (8) that are recommended to avoid floor effects in older people suggest to use the 30s CST (7) or the fastest of two chair stands at comfortable speed (20). However, even if the original test was modified to just one repetition, this floor effect would remain as none of the participants in this study who were unable to perform the CST including five repetitions, were able to complete even a single chair stand. The test might be sufficiently meaningful if test results are dichotomized in the subgroups slow/no CST and normal CST.
Moreover, the two tests detected different sub-groups as having probable sarcopenia. The EWGSOP2 advocates that both tests can be used interchangeably as estimates of strength (8). However, they may identify different determinants of muscle strength. HGS may be reflective of overall isometric strength (21) while the CST does not only measure pure muscle strength but also reflects other neuro-muscular properties such as power and balance [25, 26]. Arguably, power and balance are not normally required in nursing-home residents as the chair stand is typically performed slowly with use of arms/hands as additional support (27).
The prevalence of probable sarcopenia was almost threefold higher in this group of nursing-home residents than in community-living older people of similar age (22), and 20% higher than in nursing-home residents who were 10 years younger (23). Nursing-home residents are at particular risk of strength decline (3) due to physical inactivity and malnutrition (1). Given the negative associations of muscle weakness, such as mobility limitations and high risk of falls (1), the high prevalence of probable sarcopenia highlights the need for feasible strength assessment in these individuals to initiate adequate interventions and to avoid further decline.
Overall, both detection tests had an indicative value for gait speed and frailty, while HGS was also suggestive of overall strength and ADL performance in this study of elderly nursing-home residents.
The participants with probable sarcopenia had lower isometric elbow flexor and knee extensor strength than those without, but only when detected by low HGS. This may reflect that HGS is an indicator of general neuromuscular capacity (2, 21) whereas CST covers task-specific capabilities such as leg power (see previous section) (24, 25). Further research is needed to evaluate the relationship between HGS, the CST and different aspects of strength as well as feasible modifications of the CST for the very old population.
The sub-groups differed in gait speed, independent of test for detection by 0.2 m/s. Both tests were almost equally accurate in distinguishing gait speed. An increase of 0.1m/s has been reported to be a substantial change (26) that significantly improves survival after one year (27). Previous literature evaluating physical function in nursing-home residents reported a significant correlation between HGS and gait speed (r = 0.24, p<.001) (23). The present results, applying sarcopenia-specific cut off values for HGS (8), contribute the knowledge that people with low HGS and CST are likely to have gait speed indicating particular risk for falls and hospitalization (28). Hence, detection of probable sarcopenia as an indicator of walking speed, can be used to initiate individual gait assessment.
Regarding ADL, only low HGS could detect differences, not the CST. More people with probable sarcopenia were dependent in ADL than those without, which is consistent with findings reported in community-living older adults (22), and in people across different health care settings (5). Independence in ADL is of particular importance in older people due to its relation to quality of life and health care costs (29). Therefore, HGS had an additional value over CST in this population as it could potentially be used for frequent screening of ADL performance.
Number of comorbidities were not different between sub-groups, independent of the detection test. These findings correspond with previous literature that evaluated the relationship between HGS and the occurrence of comorbidity (≥ 3 chronic diseases) in community-living older adults (30). Hence, low HGS and CST have indicative value for physical function in older nursing-home residents independent of chronic diseases.
Both detection tests of probable sarcopenia might also be useful as screening tests for frailty status with the CST being superior to HGS, since it was more accurate in distinguishing between prefrail and frail nursing-home residents. Even though frailty symptoms occurred in all 30 participants, reflecting a very vulnerable population, the status of frailty is meaningful for adverse outcomes and mortality (16).
Limitations of the study
A limitation of this study is the small number of participants which led to small sub-groups. The findings could therefore be under/overestimated. However, since information about muscle status in nursing-home residents is rare, the findings may still provide indicative evidence for future research. Secondly, participants were only recruited from one nursing-home in Switzerland. However, important health parameters are similar across residents of nursing-homes in Europe, such as functional decline and severity of disability (3). Therefore, the results could be considered generizable to nursing-homes in this continent.
Conclusions
Muscle strength testing is crucial in older people who are at risk for strength decline, as well as for those already experiencing consequences of muscle weakness, such as functional limitations and ADL dependence. The present results provide novel, clinically relevant data about the feasibility of strength tests for nursing-home residents that can be used for detection of probable sarcopenia but also as screening tests for health outcomes. Low HGS as well as slow/no CST demonstrate high prevalence of probable sarcopenia in nursing-home residents, indicating low level of physical function and frailty. However, the CST may not be an implementable measure of strength in clinical practice of nursing-homes, hence, HGS is recommended as a routine test for detection of probable sarcopenia.
Funding: The authors received no specific funding for this work.
Acknowledgments: We would like to thank the nursing-home residents for their participation. We acknowledge Adullam Spital und Pflegezentrum Basel for providing equipment and wish to thank Dr. Hans-Jörg Ledermann for his valuable suggestions during the planning of this research work, and the nursing staff for their help in recruitment and data collection.
Conflict of interest: Julia Wearing declares that she has no competing interests. Maria Stokes declares that she has no competing interests. Rob de Bie declares that he has no competing interests. Eling de Bruin declares that he has no competing interests.
Ethical standards: Volunteers who were able to understand study content and signed informed consent, were included in the study. All study procedures complied with the principles of the Declaration of Helsinki for ethical research in humans and the study received approval from the local ethics committee (project-ID 2017-00839).
References
1. McLeod M, Breen L, Hamilton DL, Philp A. Live strong and prosper: the importance of skeletal muscle strength for healthy ageing. Biogerontology. 2016;17(3):497-510.
2. Bohannon RW. Grip Strength: An Indispensable Biomarker For Older Adults. Clin Interv Aging. 2019;14:1681-91.
3. Laffon de Mazieres C, Morley JE, Levy C, et al. Prevention of Functional Decline by Reframing the Role of Nursing Homes? J Am Med Dir Assoc. 2017;18(2):105-10.
4. Onder G, Carpenter I, Finne-Soveri H, et al. Assessment of nursing home residents in Europe: the Services and Health for Elderly in Long TERm care (SHELTER) study. BMC Health Serv Res. 2012;12:5.
5. Roberts HC, Syddall HE, Sparkes J, et al. Grip strength and its determinants among older people in different healthcare settings. Age Ageing. 2014;43(2):241-6.
6. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381(9868):752-62.
7. Beaudart C, Rolland Y, Cruz-Jentoft AJ, et al. Assessment of Muscle Function and Physical Performance in Daily Clinical Practice : A position paper endorsed by the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO). Calcif Tissue Int. 2019;105(1):1-14.
8. Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019.
9. Neumann S, Kwisda S, Krettek C, Gaulke R. Comparison of the Grip Strength Using the Martin-Vigorimeter and the JAMAR-Dynamometer: Establishment of Normal Values. In Vivo. 2017;31(5):917-24.
10. Shen Y, Chen J, Chen X, Hou L, Lin X, Yang M. Prevalence and Associated Factors of Sarcopenia in Nursing Home Residents: A Systematic Review and Meta-analysis. J Am Med Dir Assoc. 2019;20(1):5-13.
11. Shechtman O, Sindhu BS. Grip Strength. In: ASTH, editor. Clinical Assessment Recommendations, 3rd edition2013.
12. Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85-94.
13. Morris JN, Fries BE, Mehr DR, et al. MDS Cognitive Performance Scale. J Gerontol. 1994;49(4):M174-82.
14. Arnold CM, Warkentin KD, Chilibeck PD, Magnus CR. The reliability and validity of handheld dynamometry for the measurement of lower-extremity muscle strength in older adults. Journal of strength and conditioning research / National Strength & Conditioning Association. 2010;24(3):815-24.
15. Kim HJ, Park I, Lee HJ, Lee O. The reliability and validity of gait speed with different walking pace and distances against general health, physical function, and chronic disease in aged adults. J Exerc Nutrition Biochem. 2016;20(3):46-50.
16. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146-56.
17. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159-74.
18. Casas-Herrero A, Cadore EL, Zambom-Ferraresi F, et al. Functional capacity, muscle fat infiltration, power output, and cognitive impairment in institutionalized frail oldest old. Rejuvenation Res. 2013;16(5):396-403.
19. Eriksrud O, Bohannon RW. Relationship of knee extension force to independence in sit-to-stand performance in patients receiving acute rehabilitation. Physical therapy. 2003;83(6):544-51.
20. Mehmet H, Yang AWH, Robinson SR. What is the optimal chair stand test protocol for older adults? A systematic review. Disabil Rehabil. 2019:1-8.
21. McGrath RP, Kraemer WJ, Snih SA, Peterson MD. Handgrip Strength and Health in Aging Adults. Sports Med. 2018;48(9):1993-2000.
22. Franzon K, Zethelius B, Cederholm T, Kilander L. The impact of muscle function, muscle mass and sarcopenia on independent ageing in very old Swedish men. BMC Geriatr. 2019;19(1):153.
23. Wisniowska-Szurlej A, Cwirlej-Sozanska A, Woloszyn N, Sozanski B, Wilmowska-Pietruszynska A. Association between Handgrip Strength, Mobility, Leg Strength, Flexibility, and Postural Balance in Older Adults under Long-Term Care Facilities. Biomed Res Int. 2019;2019:1042834.
24. Alcazar J, Losa-Reyna J, Rodriguez-Lopez C, et al. The sit-to-stand muscle power test: An easy, inexpensive and portable procedure to assess muscle power in older people. Experimental gerontology. 2018;112:38-43.
25. Lord SR, Murray SM, Chapman K, Munro B, Tiedemann A. Sit-to-stand performance depends on sensation, speed, balance, and psychological status in addition to strength in older people. J Gerontol A Biol Sci Med Sci. 2002;57(8):M539-43.
26. Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54(5):743-9.
27. Hardy SE, Perera S, Roumani YF, Chandler JM, Studenski SA. Improvement in usual gait speed predicts better survival in older adults. J Am Geriatr Soc. 2007;55(11):1727-34.
28. Middleton A, Fritz SL, Lusardi M. Walking speed: the functional vital sign. J Aging Phys Act. 2015;23(2):314-22.
29. Mlinac ME, Feng MC. Assessment of Activities of Daily Living, Self-Care, and Independence. Arch Clin Neuropsychol. 2016;31(6):506-16.
30. Cesari M, Onder G, Russo A, et al. Comorbidity and physical function: results from the aging and longevity study in the Sirente geographic area (ilSIRENTE study). Gerontology. 2006;52(1):24-32.
