J. Cooper1, D. Powell2
1. Royal Wolverhampton Hospitals NHS Trust, United Kingdom; 2. Walsall Healthcare NHS Trust, United Kingdom
Corresponding Author: J. Cooper, Royal Wolverhampton Hospitals NHS Trust, United Kingdom, june.cooper6@nhs.net
Jour Nursing Home Res 2024;10:1-7
Published online June 6, 2024, http://dx.doi.org/10.14283/jnhrs.2024.1
Abstract
BACKGROUND: The PureWick™ System (PureWick) is intended for non-invasive urine output management in female patients. PureWick is comprised of the PureWick™ Female External Catheter and the PureWick™ Urine Collection System.
OBJECTIVES: The objectives were: (i) to observe how PureWick impacts caregivers and patients, in the care/nursing home setting and at home, and (ii) to compare PureWick with other urinary incontinence management systems (incontinence pads and Foley catheters). The impact of PureWick on care/nursing home patients was evaluated by comparing the number of incontinence interventions (pad checks, pad changes, other observations such as bed pan/bed linen/clothing change or wash) when using PureWick compared with pads or Foley catheters. Care/nursing home and home users provided Patient-Reported Outcomes Measurements (PROMs) feedback on user experience of comfort, ease of use, efficacy, impact on daily living and quality of life when using PureWick compared with pads or Foley catheters. The impact of PureWick on carers was evaluated in the PROMs questionnaire by asking how much continence care they had given the user while using PureWick compared with pads. Carers were also given the opportunity to provide any additional comments.
STUDY DESIGN, SETTING AND PARTICIPANTS: Staff in care/nursing homes identified female incontinent residents using pads or indwelling Foley catheters who met the eligibility criteria for the study. Participants (25) ranging from 31 to 107 years (mean age 75.7 years) of age enrolled in the observational study: 11 from care/nursing homes and 14 home-based users.
MEASUREMENTS: Baseline continence information was collected for 10-14 days, then for 10-14 days using PureWick. Care staff recorded the daily intervention data which was then collated. This was an observational study, and due to the sample size of 25 participants across two different data gathering approaches, statistical analysis methods were not applied.
RESULTS:Notably fewer pad-only changes were recorded during the PureWick trial compared with baseline (103 vs 522). Night-time interventions that disturb the patient reduced by 65%, with an 89% reduction in participant-reported disturbance of sleep. 88% of PROMS respondents reported that PureWick kept them dry all or most of the time, and 52% said that PureWick never caused them skin irritation. 56% reported that PureWick never caused them to worry about smell; only 16% said this of pads or catheters. PureWick was rated 7.2/10 for comfort compared to 5.7/10 for either pads or catheters.
CONCLUSIONS: PureWick has user-reported benefits (including improved independence, dignity and quality of life) over other continence products for some users. The overall opinion of PureWick was positive compared with pads, with 64% of care/nursing home users choosing to continue using PureWick beyond the trial period, and all home users choosing to continue using PureWick following their trial.
Key words: PureWick, incontinence, care, nursing, female.
Introduction
A study of women who experience urinary incontinence found that participants associated a range of negative features with urinary incontinence, including: loss of control, anxiety, sleep disturbances, a sense of negative self-perception and a reduced ability to enjoy a satisfying social life due to isolation and embarrassment (1, 2). Urinary incontinence can also lead to falls and fractures, increasing morbidity and healthcare costs (2).
Females with urinary incontinence can be negatively impacted by their use of pads or indwelling Foley catheters in a number of ways. Patients with a Foley catheter are more likely to have experienced a lower quality of life, brought about by a fear of urine leakage, odour, painful catheter changes, urinary infections, and negative effects on daily life activities (3). Furthermore, many patients experience catheter blockage and urine bypassing (4).
Individuals wearing pads that utilise super-absorbent materials, and who lie or sit for prolonged periods while the pad accumulates urine, will experience varying levels of moisture, pressure and shear acting on their skin (5). This can lead to skin irritation and pressure ulcers, which can have a significant effect on quality of life (5). Pads can be described by some women as being bulky and rough, with movement of the pad causing discomfort, whilst worries about visibility and smell are common (2).
Urinary incontinence has a negative impact on patients and their caregivers. Managing personal care, including continence care, can become the focus of daily life and the health of caregivers may be impacted (6). Providing 24-hour continence care can contribute to caregiver exhaustion and negatively impact relationships between caregiver and patient (6). The provision of continence care can be expensive for patients and caregivers, taking into account laundry-associated costs (water and electricity), containment products and cleaning materials (6).
One component of the PureWick™ System (PureWick) is a female external catheter. It has been developed for non-invasive urine output management and serves as an alternative to pads and Foley catheters. It is intended to aid undisturbed sleep, reduce the need for carer intervention at night, reduce the risk of night-time falls, keep skin dry and increase comfort.
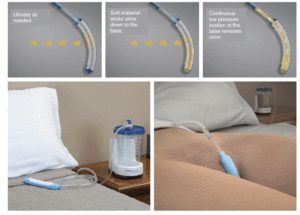
The PureWick female external catheter utilises continuous low-pressure suction to wick urine away from the body into a collection canister (7). It is required to be plugged in to a power source to provide suction, however it is available with backup battery power if required (8). PureWick can be used during the day or the night while the patient is stationary, either lying, reclining on their back or side, or when seated in a chair (7), and patients can wear mesh underwear while using PureWick (9). The PureWick system is small but does require a nightstand for the collection canister to sit on, or a small table if the patient is sat in a chair instead of in bed (8). The external catheter should be changed every 8-12 hours or if soiled (7), and the canister is reusable however it should be cleaned once daily at a minimum (9). Optional covers are provided which fit over the canister for privacy (10).
Figure 1 illustrates how PureWick works.
The suitability for PureWick varies depending on the individual patient. Included among those for whom PureWick is not suitable are those who do not have mental capacity or who have only partial mental capacity, those who struggle with agitation, aggression or confusion, those who are very active in bed and those who are too thin to keep the device well positioned between the buttocks. While not recommended for dementia patients, it could be used, if necessary, by adding net pants, provided the individual is not too active or likely to pull it out of position. Individual assessments (similar to pad assessments) should be carried out for these individuals.
The purpose of this observational study was to capture and compare information and evidence about the efficacy and benefits of PureWick with other interventions currently used in the management of urinary incontinence, and to understand how PureWick impacts carers and patients, both in a care/nursing home setting and among users in their own homes.
Methods
Process
The study was comprised of two phases:
(1) Workstream 1a was an observational study of PureWick use in the care/nursing home setting, between December 2021 and July 2022, involving pre-PureWick and during PureWick use daily intervention reporting. Care/nursing home staff identified eligible patients (see inclusion/exclusion criteria in Table 1). Consent was obtained. Baseline information for each participant was collected. Full training on the use of the PureWick female external catheter was provided to care staff. Daily continence information was captured and recorded for each participant for a period of between 10 and 14 days with current products (pads or Foley catheters), and subsequently with the PureWick female external catheter. Care staff recorded the daily intervention information, which was then collated by the nurse consultant.
Workstream 1b was the completion of anonymous Patient-Reported Outcome Measures (PROMS) questionnaires (Appendix), focusing on users’ experience of their previous incontinence products as well as PureWick, and examining their experience of comfort, ease of use, efficacy of the product and the product’s impact on their daily living and quality of life. PROMs questionnaires also included one question aimed at carers, which asked how much continence care they had given the user while using PureWick compared with pads. Carers were also given the opportunity to provide any additional comments.
(2) Workstream 2 participants were home-based PureWick users, from whom experience data were collated and analysed. PROMS questionnaires were carried out by telephone, with either users or carers at home, as for participants in care/nursing homes.
Statistical analysis
This was an observational study, and due to the sample size of 25 participants across two different data gathering approaches, statistical analysis methods were not applied.
Participant profile
Five care homes identified those female incontinent residents using pads or indwelling Foley catheters, who met the eligibility criteria (see Table 1), primarily, those who were not doubly incontinent more than twice in a 24h period, and those whose mental capacity was not likely to cause them to remove the device. Of these eligible patients, 11 completed 10 or more days of baseline and comparative PureWick data, and 17 completed only partial data (either due to failing to meet criteria or dropping out before 10 days’ use). A further 14 participants were PureWick users at home, bringing the total study enrolment to 25. Ages of participants ranged from 31 to 107 years (mean age 75.7 years): 3 were under the age of 60, 11 were between 60 and 79, 10 were between 80 and 99, and 1 was aged over 100. Of the 25 participants, only 2 were actively mobile; 14 were bedridden, two were confined to a (wheel)chair, and seven spent their time mostly in a chair. Incontinence ranged from moderate to very severe and was of a range of types: functional or mixed incontinence (the majority), urge incontinence, overflow and reflex incontinence.
Eligibility criteria
Results
Interventions
Recorded intervention types included: check if the patient was dry; check if the patient was damp but not requiring a change; pad-only change; pad change and wash; PureWick wick change only; PureWick wick change and urine collection; PureWick wick change and pad change; bed pad change; bed linen change; clothing change; wash; resident wet/dry or soiled.
Interventions numbers/frequency
Carers provided 7.7% fewer continence care interventions per 24h when participants were using PureWick as opposed to previous pad or Foley catheter.
Carer number and care home resource
The average time spent supporting a patient’s continence needs reduced by 16% when using PureWick. The average number of carers per intervention was reduced from 2.3 to 2.0, freeing time and resource for other care needs, and disturbing residents less.
Impact on type of intervention
Notably fewer pad-only changes were recorded during the PureWick trial (103) compared with baseline (522), with resulting decrease in the level of disturbance due to continence care. There was a 51% increase in the ‘check, patient dry’ intervention with PureWick compared with the same intervention check with baseline products, resulting in a growth of confidence in the PureWick effectiveness.
Carer feedback
Of 20 carers, 15 reported in their PROMS questionnaire that ‘moderately or significantly less’ continence care was required with PureWick compared with pads. Of these, eight reported ‘significantly less’.
Implications of pad and barrier cream usage
There was a reduction of 39% in the number of pad changes when using PureWick during the trial, and barrier cream applications reduced by 34% in the PureWick phase compared with baseline.
Quality of life
Sleep and night-time interventions. The average number of interventions at night-time reduced by 24% during the PureWick phase of the trial. Night-time interruptions that disturb the patient reduced by 65%, comparing aggregate of pad changes and PureWick changes between baseline and PureWick. The number of patients reporting that they were disturbed at night-time for pads and for PureWick are recorded in Table 2. In all, respondents reported a positive impact of PureWick on the quality of their night-time experience.
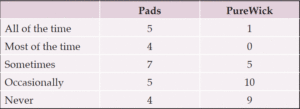
Table 2. Table comparing the number of users reporting sleep disturbance in both workstreams, between pads and PureWick
Impact on wetness
52% of PROMS respondents reported that pads never kept them dry, and 24% reported that pads kept them dry only occasionally. Comparatively, 88% of PROMS respondents reported that PureWick kept them dry either all or most of the time.
Skin irritation
When asked about skin irritation, 52% of respondents reported no irritation in the PureWick group. None of the respondents reported no irritation from pads or Foley catheters, indicating that they experienced at least some irritation.
Smell
56% of participants self-reported that they never worried about smell while using PureWick, whilst 16% reported this for pads and Foley catheters.
Comfort from continence products
PureWick was rated 7.2/10 for comfort, compared with 5.7 out of 10 for pads or Foley catheters.
Shape of continence products
80% of respondents rated PureWick either a very good or good shape, whereas 56% said this about pads or Foley catheters. None of the respondents rated PureWick a poor or very poor shape, whereas 4/25 rated pads/catheters a poor or very poor shape.
Effectiveness and overall opinion
Efficacy and leakage
Across matched days in the baseline and PureWick phases, interventions involving pad changes reduced by 39%. While 28% of respondents reported that pads/Foley catheters leaked either all or most of the time, no respondents said that PureWick leaked all of the time. 80% of respondents said that PureWick stayed in place all or most of the time, compared with 72% for pads/Foley catheters.
Overall opinion
7 of 11 participants in the care/nursing home setting chose to continue to use PureWick over pads/Foley catheters following the end of the trial, and all 14 users at home continued to use them long term.
Problematic
On a scale of 1 (not at all problematic) to 10 (very problematic), participants rated PureWick less problematic than pads/Foley catheters (2.9 vs 5.9).
Overall experience
All but one of the home long-term PureWick users reported their overall experience as excellent. Overall, the participants opinion of PureWick was favourable compared with pads.
Benefits
84% of respondents reported experiencing benefits from using PureWick.
Certain characteristics were shared by those who experienced the greatest benefit of using PureWick, which can be seen in Table 3.

Table 3. Table showing the characteristics of users who reported the greatest benefit of using PureWick
Participant/carer experience
Dignity and independence
PureWick has reduced the continence care needs for some users, giving them back some dignity and independence. It has enabled users to assume greater responsibility for their own care. As care needs are easier to manage, family are better able to care directly for users. Those users who are now able to manage their night-time incontinence may be enabled to return home from residential care. Embarrassment from the smell of pads has been eliminated. PureWick gave one user the confidence to go on holiday.
Physical and mental health
Improved skin integrity, reduced infections, and improved ability for sores to heal were all cited as positive benefits of PureWick.
Carer/family impact
PureWick has given carers and families the peace of mind of knowing that users can be left for longer periods at night without fear of finding them wet on return.
Cost
Anecdotally, home-based PureWick use resulted in reduced electricity bills as users no longer needed to wash bed linen several times a day. However, several home-based users expressed concerns about the cost of purchasing and maintaining PureWick privately rather than via prescription.
Environment
PureWick resulted in less waste being sent to landfill, and fewer problems with smell due to household bins filled with pad waste.
Discussion
The data provided by this observational study demonstrates the user-reported benefits of the PureWick external female catheter system compared with pads and indwelling Foley catheters.
Users found PureWick to be more comfortable and less irritating to skin than pads or Foley catheters. It has previously been found that periods of pressure relief and management of moisture are important for maintaining skin integrity (5). The PureWick device wicks urine away from the skin, reducing the amount of moisture that skin is exposed to for prolonged periods of time, so may better contribute to the maintenance of skin health than pads.
Female external catheters have been shown to significantly reduce the incidence of catheter-associated urinary tract infections (CAUTIs) (11). Not only do frequent urinary tract infections (UTIs) have physical, social and psychological effects on patients, but between 2020 and 2021, the cost to the NHS of antibiotic prescriptions to treat UTIs was £37 million (12, 13). Reducing the incidence of UTIs through using female external catheters could therefore potentially contribute to cost savings for healthcare systems. Although the length of this study was not adequate to assess impact on UTI incidence, home users who had been using the PureWick device long-term (more than one year), commented anecdotally that they had stopped experiencing UTIs or had experienced them less frequently since using PureWick.
Fewer interventions such as pad and bedding changes are required when using the PureWick system, meaning that patients get better quality sleep, improving their quality of life and overall wellbeing. Previously, benefits to sleep have been reported when using PureWick for both users and caregivers (14). In a 2022 study, 36% of patients who used PureWick by themselves reported improved sleep with PureWick (14). For those being taken care of by caregivers, 14% of patients reported sleep benefits, and 23% of caregivers reported sleep benefits for themselves, and feeling rested the morning after use (14). Reducing the time spent changing and washing bedding may allow family members and carers to spend more time with the user rather than on continence care.
Although cost was a concern for some users, one user reported a reduction in electricity and water bills, and less laundry powder usage due to fewer bedding changes. Additional to the utility costs to the user, incontinence products are expensive for healthcare systems, with incontinence pads alone costing the NHS roughly £80 million a year (13, 15). Compared with pads, users only need to use one PureWick external catheter per night, and the collection canister is reusable. Avoiding the continual disposal of pads may result in cost savings and may reduce the environmental impact of continence care.
Many users found PureWick most beneficial at night, and some continued to use it during the day. Reasons that some users gave for not using PureWick during the day was to be more mobile, or to keep the cost of using PureWick down. Whilst the contraindications to using PureWick have been discussed, it is also important to note that the device is intended to be used when the patient is seated, reclining, or lying in bed, and does require a stable surface for the collection canister to rest on. This may mean that PureWick is not suitable for those who would want to be more mobile (for example using an electric wheelchair) and this may warrant further research.
The authors have observed from their experience in clinical practice that PureWick may be particularly valuable for two groups of patients: younger patients, and those in end-of-life care. Younger patients who are incontinent and reliant on frequent intermittent catheterisation may benefit from being able to empty their bladder prior to outings without the risk of incomplete voiding of the bladder and infection, reducing the likelihood of distressing public incontinence episodes. PureWick may reduce some of the stigma associated with incontinence in young people. Palliative care patients who are bedbound can also benefit from the PureWick device, as it reduces the need for rolling them in bed to change pads and bedding.
Study limitations
Outcomes and learnings here reported result from this observational study only, reflect the experience of a relatively limited participant group and should not be considered exhaustive.
A detailed report of findings from this study is available upon request.
Conclusion
A total of 25 participants contributed data to the study: 11 were residents of care/nursing homes and provided comparative interventions date and PROMS feedback; 14 were home-based PureWick users and contributed PROMS experience feedback.
Interventions data showed that staff and carers spent less time and resource delivering continence care to PureWick users. Many users found PureWick most beneficial at night-time, and some used pads during the day when they wanted to be more mobile. PROMS data provided clear evidence of benefits, with users reporting increased levels of comfort, dryness and sleep quality, and most deciding to continue use of PureWick after the trial.
Those who did not have a successful experience with PureWick may have had only partial mental capacity, and struggled with agitation, confusion, or aggression, being very active in bed or being too thin to maintain positioning of the device.
Conflicts of interest: No conflicts of interest concerning this article, including financial, consultant, institutional and other relationships
Assistance with article: Medical writing support was provided by Cogora (London, United Kingdom)
Funding statement: Funding for the medical writing was provided by Becton Dickinson. The content of the publication has been led and driven by the authors with BD having limited input.
Ethical standards: All participants took part anonymously to support full disclosure and neutrality of feedback, and no identifiable data were captured. Patients were consented by either the care home or Monmouth’s nurse consultant, where the participant had mental capacity to do so. Where participants did not have capacity, consent was obtained from next of kin.
Open Access: This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
References
1. Aguilar-Navarro S, Navarrete-Reyes AP, Grados-Chavarria BH, et al. The Severity of Urinary Incontinence Decreases Health-Related Quality of Life among Community-Dwelling Elderly. J Gerontol A Biol Sci Med Sci 2012;67:1266-1271. doi: 10.1093/gerona/gls152.
2. Fu Y, Jackson C, Nelson A, et al. Exploring support, experiences and needs of older women and health professionals to inform a self-management package for urinary incontinence: a qualitative study. BMJ Open 2023;13:e071831. doi: 10.1136/bmjopen-2023-071831.
3. Wiedemann A, Gedding C, Heese M, et al. Lebensqualität bei Trägern eines suprapubischen oder transurethralen Harnblasenkatheters als lebenslange Dauerversorgung. Urologe 2022;61:18-30. doi: 10.1007/s00120-021-01642-1
4. Rew M, Woodward S. Troubleshooting common problems associated with long-term catheters. British Journal of Nursing 2001;10:764-774. doi: 10.12968/bjon.2001.10.12.5302.
5. Bostan LE, Worsley PR, Abbas S, et al. The influence of incontinence pads moisture at the loaded skin interface. J Tissue Viability 2019;28:125-132. doi: 10.1016/j.jtv.2019.05.002.
6. Gove D, Scerri A, Georges J, et al. Continence care for people with dementia living at home in Europe: a review of literature with a focus on problems and challenges. J Clin Nurs 2017;26:356-365. doi: 10.1111/jocn.13582.
7. Becton Dickinson. BD PureWick Patient Education Brochure. Available at: https://bit.ly/3vNc1BJ. Accessed April 3, 2024.
8. Becton Dickinson. How the PureWickTM System Works. 2022. Available at: https://bit.ly/4cMek8K. Accessed April 3, 2024.
9. Becton Dickinson. Clinician FAQs About PureWickTM. Available at: https://bit.ly/3PQPon3. Accessed April 3, 2024.
10. Becton Dickinson. How to Set Up Your PureWickTM System. Available at: https://bit.ly/4cHYr3k. Accessed April 3, 2024.
11. Zavodnick J, Harley C, Zabriskie K, et al. Effect of a Female External Urinary Catheter on Incidence of Catheter-Associated Urinary Tract Infection. Cureus 2020. doi: 10.7759/cureus.11113.
12. OpenPrescribing. Prescription data for section code 050103 (Urinary Tract Infection), 22 (incontinence Appliances, 2102 (Catheter) and 2113 (catheter maintenance) from April 2019 – June 2021 date. Available at: https://bit.ly/4arKvZJ. Accessed April 4, 2024.
13. Bladder Interest Group. The Cost of Poor Bladder Management Report. 2021. Available at: https://bit.ly/3VN7cDd. Accessed April 5, 2024.
14. Khosla L, Sani JM, Chughtai B. Patient and caretaker satisfaction with the PureWick system. Can J Urol 2022;29:11216-11223.
15. NHS England. Excellence in Continence Care: Practical guidance for commissioners, and leaders in health and social care. 2018.
The Author(s) 2024