N. Viveky1, L. Thorpe2, J. Alcorn1, T. Hadjistavropoulos3, S.J Whiting1
College of Pharmacy & 1. Nutrition, 2. College of Medicine, University of Saskatchewan, 3. Department of Psychology, University of Regina; Corresponding author: Lilian Thorpe, MD, PhD. Department of Community Health and Epidemiology, College of Medicine, 104 Clinic Place, Saskatoon, SK, S7N 2Z4, Canada; Phone: (306)966-7977 (alternate 306-655-7997), Fax: (306)966-7920, Email: lilian.thorpe@usask.ca
Abstract
The anti-inflammatory and anti-oxidant properties of flaxseed lignans could benefit age-related chronic conditions. We aimed to examine the safety of supplementation of the major flaxseed lignan, secoisolariciresinol diglucoside (SDG) in frail older adults residing in long-term care (LTC) homes. Twenty-six older adults (60-80 years of age) met the inclusion criteria and were enrolled in a double blind randomized control trial of SDG supplementation at 300 mg/day for six months. Adverse events were recorded every week along with other blood and functionality tests. Participants in the treatment group demonstrated no evidence of hypoglycemia and hypotension or other adverse events. We conclude that SDG supplementation (300 mg/day) in a frail, complex patient population causes no significant adverse outcomes.
Key words: Flaxseed, safety, hypoglycaemia, hypotension, older adults, long-term care.
Introduction
Flaxseed may contribute to protection from cardiovascular disease. Flaxseed supplementation has been shown to result in modest reduction in the plasma levels of total cholesterol and low-density lipoprotein cholesterol in both normal and hypercholesterolemic patients (1). Consumption of 30 g milled flaxseed, equivalent to approximately 450 mg secoisolariciresinol diglucoside (SDG) (2), for six months in adults over 40 years resulted in a significant decrease in both systolic and diastolic blood pressure, and the effect was more pronounced in hypertensive patients (3). Postmenopausal women consuming 40 g of ground flaxseed (approximately 600 mg SDG) for 3 months showed reduction in serum total cholesterol, non-high-density lipoprotein cholesterol, low-density and high-density lipoprotein cholesterol, and triglycerides (4). Both lignan and dietary fibre of flaxseed demonstrate hypocholesterolemic action, anti-proliferative effects, and anti-atherogenic and anti-inflammatory potential, mechanisms that provide protective effects in the management of chronic conditions such as metabolic syndrome (5,6). After ingestion, human gut microflora converts the plant lignans into mammalian lignans, also called phytoestrogens (7).
Investigation of the health benefits of flaxseed and of its bioactive components is important to pursue. Older adults with underlying health conditions, particularly those residing in long-term care (LTC) home, could benefit from the properties of flax. A study where 30 g of milled flaxseed was given to older adults (45-64 years) showed benefits related to inflammation and aging (8). A Canadian survey showed high prevalence of chronic conditions in older adults requiring care, including arthritis/rheumatism (47%), back problems and cataracts or glaucoma (25%), and heart disease (20%) (9). Yet older adults undergo physiological changes in organ function, which may impact the bioavailability and disposition of flaxseed bioactive components relative to the young adult. Consequently, a need exists to examine the effects of flaxseed supplementation in this population.
We have previously reported no incidence of adverse effects of a high dose of flaxseed lignan, 543 mg/d of SDG, from a community-based efficacy trial in healthy community living older adults (6). No adverse effects on blood glucose and blood pressure were noted with this flaxseed lignan dose (10). However, in older adults who are frail and have underlying health problems, the ability of SDG to cause reductions in blood glucose (11) and blood pressure levels (6) may pose a health risk that warrants an investigation of the safety of flaxseed lignan in frail LTC residents. As such, a double blind placebo controlled study was designed to address the safety of 300 mg flaxseed lignan (SDG) supplementation in older adults who were frail and had chronic health conditions.
Methods
Twenty six LTC residents, 60 to 80 years of age who were not at undue risk of adverse events, were enrolled in the study. Enrollment criteria also included residing in LTC for at least four weeks. In the absence of previous safety studies with this population, Health Canada required restrictive inclusion criteria to ensure avoidance of any undue risk of a significant adverse event. These requirements excluded residents with any contraindication to receiving vitamin D, intolerances or allergies to flax, participation in any other clinical trial with an investigational agent within a month prior to randomization, severe cognitive impairment that would prevent them from participating in neuropsychological testing, those with clinically significant abnormal laboratory test results and any with expected life expectancies shorter than one year based on medical opinion. Medical exclusions also removed potential participants with a diagnosis of cancer within the past 2 years, (if female) a family or personal history of breast cancer or ovarian cancer, symptomatic or at significant risk of hypotension, unstable diabetes or diabetes with insulin treatment, fasting hypoglycemia, significant or unstable liver or kidney disorder, unstable or severe cardiac disease or myocardial infarction in the past 6 months, stroke in the past 6 months (or had one previously which significantly affected his or her physical mobility), current bleeding conditions or at significant risk of bleeding, migraine with aura within the last year, significant immunocompromise or any other specific health conditions or other unstable medical disease including, but not limited to, genitourinary disorder, pulmonary disorder, epilepsy and gastrointestinal disorder (including inflammatory bowel disease but excluding constipation). Excluded also were residents taking warfarin, clopidogrel, ticlopidine, dipyridamole or their analogues, hormone replacement therapy and any likely to require prohibited concomitant therapy during the trial. Ethics clearance and consent was obtained as per the requirements of our institutional research ethics board. Blood collection, vital signs, chart review, and individual assessments were performed at baseline, week 12, and week 24 by trained study personnel.
Even after applying these restrictive exclusion criteria most remaining subjects had multiple health conditions such as mild hypertension, arthritis, moderate dementia, and type 2 diabetes suggesting a more frail population than the general population. After consenting, eligible participants were randomly assigned to an intervention or a control group. Participants in the intervention group received SDG-enhanced (38%) food grade flax lignan complex available as BeneFlax (Archer Daniel Midlands, Natural Health Products File # OF2-31-3-13412-2-4.) at a dose of 300 milligrams (438µmoles) SDG/per day (total BeneFlax was 0.8 g) plus 1000 IU vitamin D3 for 6 months given as an NHP-approved supplement available through the pharmacist in charge of that LTC long-term care (LTC) home. Participants in the Placebo Control received 0.3 g whey protein (equivalent volume to BeneFlax) in identical packaging, plus 1000 IU vitamin D3. The lignan or whey was added to a tablespoon of apple sauce or equivalent food by nursing staff and administered along with other medications ordered for that subject.
The study (Clinical trial registration number: NHPD-150212) had both a safety and an efficacy hypothesis. For efficacy, we hypothesized that the consumption of SDG in older frail adults would decrease oxidative stress and associated inflammation. The primary outcome measure was biomarkers of inflammation. Secondary outcome measures included oxidative stress biomarkers, functional outcomes including mental functioning, locomotion, and pain, blood pressure, bone loss, and glycemic control. Our safety hypothesis was that daily SDG (300 mg) for six months would cause no adverse effects in frail older adults. The primary outcome measure was incidence of adverse events. Secondary outcome measures included physical measures (vital signs, weight, waist circumference, calf circumference, arm circumference), risk of hypotension, risk of hypoglycemia, and laboratory investigations (urea, creatinine, bilirubin, platelets, hematocrit, hemoglobin, MCH, MCV, WBC, AST, ALT, ALP, protein, glucose).
Safety assessments included weekly documentation of adverse experiences, falls, and mortality. Individual falls history prior to enrolment was charted as 0 (none), 1 (occasional), and 2 (frequent). Systolic hypotension was defined as less than 80 mm Hg. Orthostatic hypotension was defined as reduction of systolic blood pressure of at least 20 mm Hg or diastolic blood pressure of at least 10 mm Hg within 3 minutes of standing after restful sitting for at least five minutes. If participants were unable to stand, we collected lying and sitting blood pressures. Hypoglycemia based on an individual test score was defined as a fasting glucose measured below the laboratory reference range (3.6-6.0 mmol/L). Clinically significant individual hypoglycemia was defined as: fasting glucose below the laboratory reference range and documented clinical symptoms such as dizziness or shakiness that required nursing intervention or a change in prescribed hypoglycemic agents.
Results
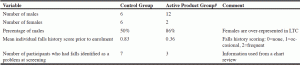
Table 1 shows sex distribution as well as fall history prior to beginning the study. A total of 26 participants were enrolled in the study (18 male and 8 female). The subject numbers reflected the exclusion criteria, which reduced the number of eligible participants. Of residents below the age of 80 y, many had considerable health challenges resulting in their original admission to LTC, many of which resulted in their exclusion from this study.
# Secoisolariciresinol diglucoside (SDG)-enhanced (38%) food grade flax lignan complex at a dose of 300 milligrams (438µmoles) SDG/per day
Of the enrolled participants, two withdrew prematurely (one discontinued prior to the end of the study, and 3 died prior to the end of the study). The falls history was 0.83 in control group and 0.36 in active product group. The mean age of participants was 71 years. Numerous adverse events typical of residents in LTC (falls, behavioural problems, poor dietary intake, and incontinence) were reported throughout the study for both study groups, with not much difference amongst the placebo and active treatment group. One participant developed loose stools within a few days of starting the active product, which resolved and remained resolved after the product was discontinued. As episodic loose stools are common, it is not clear whether SDG was responsible for this. However, this event was coded as “probably related” and the participant was withdrawn.
Blood pressure, pulse, and blood glucose are listed in Table 2. Mean diastolic and systolic blood pressure before and 2-4 hours after ingestion of first dose of SDG as well as mean blood glucose were normal. One participant had fasting blood glucose below the laboratory reference range («laboratory hypoglycemia»), but this occurred before the active phase of the study and was not accompanied by clinical symptoms or change in medical or nursing management (i.e., was not “clinically significant hypoglycemia») or change in medications or diet. Subsequent tests were normal. No participant had episodes of systolic hypotension or orthostatic hypotension during the study period and none of the participants showed evidence of changes in pulse rate, systolic and diastolic blood pressure, or an orthostatic drop in blood pressure from lying to standing (or sitting) position.
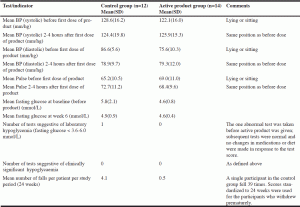
Table 2 Absence of adverse outcomes related to safety: blood pressure, heart rate, blood glucose and incidence of falls in the LTC residents enrolled in the study
# Secoisolariciresinol diglucoside (SDG)-enhanced (38%) food grade flax lignan complex at a dose of 300 milligrams (438µmoles) SDG/per day
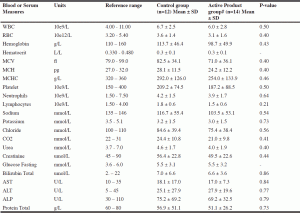
Mean biochemical measures including WBC, RBC, hemoglobin, hematocrit, MCV, MCH, MCHC, platelets, neutrophils, lymphocytes, sodium, potassium, chloride, CO2, urea, creatinine, glucose fasting, bilirubin total, AST, ALT, ALP, and protein total were in the normal reference range (Table 3) for both the groups. There was no significant difference between the placebo and active treatment group at the end of study depicting safety of the BeneFlax supplementation.
P value significant at ≤ 0.05; # Secoisolariciresinol diglucoside (SDG)-enhanced (38%) food grade flax lignan complex at a dose of 300 milligrams (438µmoles) SDG/per day
Discussion
The results of this study along with our previous findings (10) suggest that people with hypertension could be included in such future intervention studies. All participants tolerated the product well. Although statistical testing was not possible due to small sample size, which is the study limitation, descriptive data review suggested that neither falls frequency, other injuries, or patient death appeared associated with the SDG intervention.
Despite restrictive enrollment criteria (as per Health Canada requirements), our participants did still have multiple health challenges and comorbidities, which allows us to generalize our findings to most frail seniors. The current study demonstrates safety of flaxseed lignan administration in this subpopulation, which should facilitate future trials involving this population with less restrictive exclusion criteria. Thus, these data may be combined with our previous study (10) to indicate flax lignan in doses of 300-550 mg per day does not appear to pose a health risk to older adults, whether community dwelling or frail and residing in LTC.
Conclusion
The focus of this study was to examine the adverse effects of chronic flaxseed lignan supplementation at dose of 300 mg/day in the form of BeneFlax in older adults residing in LTC. Results suggest there are no concerns for hypoglycaemia, hypotension, abnormal biochemical blood measures or other adverse effects with lignan supplementation. We therefore conclude that lignan (SDG) supplementation at 300 mg/day can be safely administered to most frail older adults. Our findings have the potential of facilitating future trials involving this population, given that safety information concerning lignan supplementation has now been added to our knowledge base. This safety information suggested that broader samples of participants can be recruited in future trials.
Conflict of interest: On behalf of all authors, the corresponding author states that there is no conflict of interest. This research was funded, in part, through a team grant from the Saskatchewan Health Research Foundation.
Ethical Standards: Researchers complied with research standards mandated by the University of Saskatchewan Responsible Conduct of Research policy.
References
1. Bassett CM, Rodriguez-Leyva D, Pierce GN. Experimental and clinical research findings on the cardiovascular benefits of consuming flaxseed. Appl Physiol Nutr Metab 2009; 34(5):965-974.
2. Muir AD. Flax Lignans-Analytical Methods and How They Influence Our Understanding of Biological Activity. J AOAC Int 2006; 89(4):1147-1157.
3. Rodriguez-Leyva D, Weighell W, Edel AL, et al. Potent Antihypertensive Action of Dietary Flaxseed in Hypertensive Patients. Hypertension 2013; 62:1081-1089.
4. Lucas EA, Wild RD, Hammond LJ, et al. Flaxseed improves lipid profile without altering biomarkers of bone metabolism in postmenopausal women. J Clin Endocrinol Metab 2002; 87:1527-1532.
5. Adolphe JL, Whiting SJ, Juurlink BH, et al. Health effects with consumption of the flax lignan secoisolariciresinol diglucoside. Br J Nutr 2010; 103:929-938.
6. Cornish SM, Chilibeck PD, Paus-Jennsen L, et al. A randomized controlled trial of the effects of flaxseed lignan complex on metabolic syndrome composite score and bone mineral in older adults. Appl Physiol Nutr Metab 2009; 34:89-98.
7. Carreau C, Flouriot G, Bennetau-Pelissero C, Potier M. Enterodiol and enterolactone, two major diet-derived polyphenol metabolites have different impact on ERalpha transcriptional activa- tion in human breast cancer cells. J Steroid Biochem Mol Biol 2008; 110:176–185.
8. Caligiuri SPB, Aukema HM, Ravandi A, Pierce GN. Elevated levels of pro-inflammatory oxylipins in older subjects are normalized by flaxseed consumption. Exp Gerontol 2014; pii: S0531-5565(14)00126-0. doi: 10.1016/j.exger.2014.04.005.
9. Gilmour H, Park J. Dependency, chronic conditions and pain in seniors. Health Reports / Statistics Canada, Canadian Centre for Health Information 2006; 16:21-31.
10. Billinsky J, Glew RA, Cornish SM, et al. No evidence of hypoglycemia or hypotension in older adults during 6 months of flax lignan supplementation in a randomized controlled trial: A safety evaluation. Pharm Biol 2013; 51(6): 778-782
11. Pan A, Sun J, Chen Y, et al. Effects of a flaxseed-derived lignan supplement in type 2 diabetic patients: A randomized, double-blind, cross-over trial. PLoS One 2007; 2:e1148, 1-7.