A.K. Gergen1, P. Hosokawa2, C. Irwin1, M.J. Cohen1, F.L. Wright1, C.G. Velopulos1, E.J. Kovacs1, R.C. McIntyre Jr1, J.-P. Idrovo1
1. University of Colorado School of Medicine, Department of Surgery, Division of GI, Trauma, and Endocrine Surgery, Aurora, USA; 2. Adult and Child Consortium for Health Outcomes Research (ACCORDS), Aurora, USA. Corresponding author: Anna K. Gergen, University of Colorado School of Medicine, Department of Surgery, Division of GI, Trauma, and Endocrine Surgery, 12631 E. 17th Avenue, MS C-302, Aurora, CO 80045, USA, Telephone: 206-384-7837, Fax: 303-724-2806, Email: anna.gergen@cuanschutz.edu
Jour Nursing Home Res 2021;7:47-54
Published online September 10, 2021, http://dx.doi.org/10.14283/jnhrs.2021.8
Abstract
Objectives: Elderly patients requiring emergency general surgery (EGS) are at high risk for complications due to preexisting malnutrition. Thus, correcting nutritional deficits perioperatively is essential to improve outcomes. However, even in patients unable to tolerate enteral nutrition, initiation of parenteral nutrition (PN) is often delayed due to concerns of associated complications. In this study, we hypothesized that in elderly EGS patients with relative short-term contraindications to enteral nutrition, early administration of PN is as safe as delayed administration. Furthermore, early PN may improve outcomes by enhancing caloric intake and combatting malnutrition in the immediate perioperative period. Design and Setting: A single-institution, retrospective review was performed at a quaternary academic medical center. Participants: Participants consisted of 58 elderly patients >65 years of age admitted to the EGS service who required PN between July 2017 and July 2020. Measurements: Postoperative outcomes of patients started on PN on hospital day 0-3 (early initiation) were compared to patients started on PN on hospital day 4 or later (late initiation). Bivariate analysis was conducted using the Chi-square or Fisher’s exact test for categorical variables and the Wilcoxon-Mann-Whitney test and F-test for continuous variables. Results: Fifty-eight patients met inclusion criteria, with 27 (46.6%) patients receiving early PN and 31 (53.4%) receiving late PN. Both groups shared similar baseline characteristics, including degree of frailty, body mass index, and nutritional status at time of admission. Complications associated with PN administration were negligible, with no instances of central venous catheter insertion-related complications, catheter-associated bloodstream infection, or factors leading to early termination of PN therapy. A significantly higher proportion of patients in the early administration group met 60% of their caloric goal within 72 hours of admission (62.9% versus 19.5%, p=0.0007). Patients receiving late PN demonstrated a significantly higher rate of unplanned admission to the intensive care unit (38.7% versus 14.8%, p=0.04). Moreover, there was a 21.5% reduction in mortality among patients in the early initiation group compared to patients in the late initiation group (33.3% versus 54.8%, p=0.10). Conclusions: Early initiation of PN in hospitalized elderly EGS patients was not associated with increased adverse events compared to patients undergoing delayed PN administration. Furthermore, patients receiving early PN demonstrated a 2.6-fold decrease in the rate of unplanned admission to the intensive care unit and trended toward improved mortality. Based on these results, further prospective studies are warranted to further explore the safety and potential benefits of early PN administration in elderly surgical patients unable to receive enteral nutrition.
Key words: Elderly, geriatric, emergency general surgery, surgery, parenteral nutrition.
Introduction
Following emergent surgery, adequate nutritional status is fundamental to a patient’s successful recovery. The added stress of the perioperative period and critical illness increase caloric demands and subsequent risks for malnutrition, immune system dysfunction, and poor healing (1). Moreover, due to the nature of their disease process, most patients requiring emergency general surgery (EGS) frequently present at admission with a recent history of inadequate food intake, thus increasing their risk of prolonged undernutrition and concomitant surgical complications.
Although enteral nutrition (EN) remains the preferred route of dietary support in hospitalized patients, EGS patients frequently exhibit relative contraindications or poor tolerance to EN in the perioperative period. In the postoperative adult patient unable to receive EN, current guidelines by the American Society of Parental and Enteral Nutrition (ASPEN) favor waiting 5-7 days prior to the initiation of parenteral nutrition (PN), and only if the duration of therapy is anticipated to exceed at least 7 days (2). Even in critically ill patients with contraindications to enteral feeding for the indeterminate future, administration of PN is commonly delayed due to historical concerns over accompanying complications, such as infections, metabolic derangements, and liver dysfunction (3). While an average, previously healthy adult may tolerate delayed nutritional supplementation, the elderly population represents a unique cohort at high risk for poor postoperative outcomes due to increased rates of preexisting malnutrition, decreased baseline functional status, and diminished ability to adapt to metabolic stress (4). Consequently, these patients may exhibit a more difficult recovery in the setting of inadequate nutritional intake.
Compared to younger age groups, elderly patients account for a disproportionate burden of admissions and health care spending, presenting with a greater number of comorbid conditions, more frequent hospitalizations, and longer durations of stay (5). Furthermore, with an increasingly aging population, the percentage of people 65 years and older is expected to nearly double worldwide over the next three decades, signifying an anticipated parallel increase in health care needs (6). The need for emergency surgery increases with age, with numerous EGS diagnoses occurring at a higher incidence among older age groups (7-9). Notably, nutritional derangements in elderly patients have been associated with a broad range of unfavorable outcomes, including longer hospital stay, pressure ulcers, higher infection rates, increased falls, and death (10-12). Furthermore, malnutrition is a well-established but often underreported risk factor for poor surgical outcomes, with surgical stress creating a catabolic state that further compounds protein and energy depletion (13). Studies indicate that nearly 50% of elderly hospitalized patients are undernourished or malnourished on admission, with current guidelines recommending initiation of PN as soon as possible in critically ill patients unable to receive EN who are at high nutrition risk or severely malnourished (14, 15). However, despite the wide availability of validated nutrition assessment tools, routine screening of hospitalized patients is broadly underutilized in clinical practice (16). In addition, energy goals are frequently not met among inpatients (16, 17). Therefore, given the high incidence of preoperative malnutrition in elderly adults in conjunction with the added physical stress of surgery, it is likely that patients at high nutritional risk with indications for early PN remain grossly underestimated. This ultimately leads to delays in nutrition delivery and the inability to achieve nutritional intake goals.
Current recommendations guiding the use of PN in hospitalized patients are based on studies that include a wide range of ages and may be less pertinent to high-risk age groups, such as geriatric patients. Furthermore, while modern therapeutic guidelines, advanced monitoring, and improvements in medications have significantly decreased the overall incidence of complications associated with PN use, hesitancy regarding its initiation continues to exist among inpatient providers (18-21). Based on these observations, we hypothesized that in elderly EGS patients with relative short-term contraindications to EN, early administration of PN is as safe as delayed administration. Moreover, early administration of PN may be beneficial in elderly surgical patients by enhancing caloric intake in the early perioperative period.
Methods
Patient population and outcomes
This study was submitted to the Colorado Multiple Institutional Review Board (COMIRB) who deemed it exempt. A single-institution, retrospective review of elderly adult patients admitted to the EGS service at a quaternary academic medical center between July 2017 and July 2020 was conducted. Patients >65 years of age who required initiation of PN during admission were included. Early initiation of PN was defined as within the first 0-3 days of admission, while late initiation of PN was defined as day 4 or later. Patient data, including demographics, medical comorbidities, Charlson Comorbidity Index, protein-calorie malnutrition present on admission, body mass index, admitting diagnosis, surgical interventions, hospital length of stay, PN-related complications, 30-day postoperative complications, and 90-day mortality, were collected from patient medical records. The Charlson Comorbidity Index predicts 10-year survival based on the presence of various predefined comorbidities (22). Each comorbidity category has an associated weight based on the severity of disease and adjusted risk of mortality, with the sum of all weights resulting in a single comorbidity score. The Charlson Comorbidity index was used as an objective measure to help risk stratify patients and compare severity of illness across groups. A diagnosis of protein-calorie malnutrition was established by the consulting dietitian based on criteria provided in Supplemental Material 1. All data were stored in a REDCap database.
The primary outcome of the study was adverse events associated with PN administration, including central venous catheter insertion-related complications (pneumothorax, hemothorax, arterial injury, or nerve injury), catheter-associated bloodstream infection, catheter-associated deep venous thrombosis, and early termination of PN therapy due to associated laboratory derangements or other related complication. Secondary outcomes included hospital length of stay, days of mechanical ventilation, infection (pulmonary, non-catheter-associated bloodstream, urinary tract, wound, or other), wound dehiscence, non-catheter-associated deep venous thrombosis, pulmonary embolism, renal failure requiring renal replacement therapy, unplanned intubation, unplanned admission to the intensive care unit (ICU), unplanned reoperation, readmission within 30 days of discharge, and 90-day mortality.
Frailty Index
Frailty is a multifactorial syndrome prevalent among elderly adults and associated with various poor health outcomes, including death (23). There are many definitions and methods for measuring frailty, including a clinical phenotypic framework or calculation of a Frailty Index (FI) (24, 25). The FI is based upon the accumulation of age-related conditions and calculated by dividing the number of deficits present by the number of total deficits considered in that particular scale. The optimal approach to characterizing frailty in the geriatric acute care surgery population has been inadequately studied. We pooled deficits from multiple studies to create a list of variables that were biologically sensible, related to the process of aging, impactful in the setting of acute illness or emergent surgical intervention, and relatively easy to glean from the medical record (26, 27). A FI was calculated for each patient using the variables provided in Table 1. Vitals and laboratory variables were documented based on the first available values within 48 hours of presentation to the hospital. Based on prior reports, patients with a FI >0.25 were considered to be frail, while patients with a score of <0.08 were deemed to be non-frail (26, 27).
Modified from Cheung A, Haas B, Ringer TJ, McFarlan A, Wong CL. Canadian Study of Health and Aging Clinical Frailty Scale: Does It Predict Adverse Outcomes among Geriatric Trauma Patients? J Am Coll Surg. 2017;225(5):658-65.e3
Statistical Analysis
Bivariate analysis was conducted using the Chi-square or Fisher’s exact test for categorical variables and the Wilcoxon-Mann-Whitney test and F-test for continuous variables. Composite morbidity was defined as the presence of one or more of the following: infection, wound dehiscence, renal failure requiring renal replacement therapy, unplanned intubation, and unplanned admission to the ICU. Multivariable logistic regression analysis was used to determine variables independently associated with 90-day mortality or composite morbidity, with candidate variables including early versus late initiation of PN, age, gender, FI, body mass index, Charlson Comorbidity Index, and the number of operations.
Descriptive statistics are presented as absolute numbers and percentages for categorical variables and median and interquartile range (IQR) for continuous variables. A two-sided p-value of <0.05 was considered statistically significant. All analyses were performed using Stata version 15.1 (StatCorp, College Station, TX) and SAS version 9.4 (SAS Institute Inc, Cary, NC).
Results
Patient characteristics
A total of 58 elderly patients were admitted to the EGS service at a quaternary academic medical center and required PN initiation during the study period. Admitting diagnoses are summarized in Table 2. Intestinal obstruction (36.2%), perforated viscus (19.0%), and mesenteric ischemia (17.2%) were the three most common reasons for admission, accounting for over 70% of all diagnoses. During hospitalization, 27 (46.6%) patients received early PN, and 31 (53.4%) received late PN. Baseline characteristics of patients in the early versus late initiation groups are summarized in Table 3. Bivariate analysis revealed no significant differences between groups. The median age of the entire cohort was 71.7 years, with a relatively equal distribution of males (53.4%) and females (46.6%). The median Charlson Comorbidity Index of the entire cohort was 4, correlating to a 10-year survival of approximately 53%. Additionally, the median FI score was 0.27, suggesting a significant degree of frailty among all patients studied.
Values are presented as N (%)
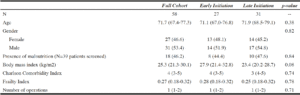
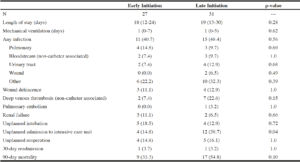
Table 3
Baseline patient characteristics of elderly patients receiving early versus late parenteral nutrition
Values are presented as N (%) or median (IQR).
At the time of admission, a consulting dietitian screened patients for the presence of protein-calorie malnutrition. Screening was completed in 67.2% of the study cohort, with the remaining patients unable to be adequately assessed. Of those screened, 46.2% of patients met criteria for moderate or severe malnutrition. There were no significant differences in malnutrition rates (44.4% versus 47.6%, p=0.84) or median body mass index (27.9 kg/m2 versus 23.4 kg/m2, p=0.08) between early and late administration groups.
Most patients (91.4%) required 1 or more operations, while the remaining 8.6% of patients were admitted to the EGS service for observation and non-operative management. Of those undergoing surgery, 64.2% required a small and/or large bowel resection, and 41.5% were left with an open abdomen after the initial operation with return to the operating room at a later date for abdominal closure.
Outcomes following early versus late initiation of parenteral nutrition
The incidence of adverse events explicitly associated with PN administration was overall rare. There were no instances of central venous catheter insertion-related complications, including pneumothorax, hemothorax, arterial injury, or nerve injury, or catheter-associated bloodstream infections in either the early or late initiation groups. In addition, there were no cases requiring early termination of PN therapy for any reason. The rate of catheter-associated deep venous thrombosis was similar between early and late initiation groups (14.8% versus 9.7%, p=0.69). Notably, a significantly higher proportion of patients in the early administration group met 60% of their caloric goal within 72 hours of admission (62.9% versus 19.5%, p=0.0007).
Other postoperative complications, including infection (40.7% versus 48.4%, p=0.56), wound dehiscence (11.1% versus 12.9%, p=1.0), renal failure (11.1% versus 6.5%, p=0.66), unplanned intubation (18.5% versus 12.9%, p=0.72), unplanned reoperation (14.8% versus 16.1%, p=1.0), and 30-day readmission (3.7% versus 3.2%, p=1.0) were similar between early and late initiation groups (Table 4). Likewise, length of stay (18 days versus 19 days, p=0.28) and days of mechanical ventilation (1 day versus 1 day, p=0.62) did not significantly differ. The early initiation group demonstrated a significant decrease in critical care needs, with a 23.9% reduction in the rate of unplanned admission to the ICU (14.8% versus 38.7%, p=0.04). Moreover, there was a 21.5% reduction in mortality rate among patients in the early initiation group compared to patients in the late initiation group (33.3% versus 54.8%, p=0.10).
Values are presented as N (%) or median (IQR).
In a multivariable logistic regression model, timing of initiation of PN was not a significant predictor of 90-day mortality (Table 5). Charlson Comorbidity Index and number of operations were the only factors independently predictive of mortality. Specifically, for each 1-point increase in Charlson Comorbidity Index, a patient’s chance of death doubled (OR 2.09, 95% CI 1.24-3.51, p=0.006). Likewise, for each additional operation endured, a patient’s chance of death increased 2.5 times (OR 2.56, 95% CI 1.28-5.15, p=0.001). A multivariable logistic model for overall morbidity, including aggregated events of any infection, wound dehiscence, renal failure, unplanned intubation, or unplanned admission to the ICU, revealed no significant predictors.
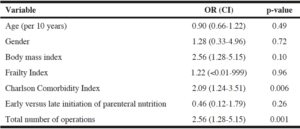
Table 5
Multivariable logistic regression model of variables independently associated with 90-day mortality
OR: odds ratio; CI: confidence interval.
Discussion
EGS patients frequently demonstrate relative contraindications to EN in the perioperative period, and thus, administration of PN is often a consideration. However, controversy and variation in clinical practice persist regarding the optimal timing of initiation of PN in the postoperative patient. Elderly surgical patients represent a particularly vulnerable cohort of patients at increased risk for malnutrition and postoperative outcomes. PN is frequently delayed due to concerns regarding PN-associated complications. The goal of this study was to evaluate whether early administration of PN in hospitalized elderly EGS patients was associated with increased adverse events compared to patients receiving delayed PN. Our results indicate that early initiation of PN is as safe as delayed initiation in elderly surgical patients with short-term contraindications to enteral feeding. Furthermore, patients receiving early PN demonstrated a 2.6-fold decrease in the rate of unplanned admission to the ICU and trended toward improved mortality, indicating decreased critical care needs and potentially reduced severity of illness.
While the timing of initiation of PN in adult patients has been frequently debated in the literature, there is continued controversy over optimal administration practices and a lack of data specifically addressing PN use in the elderly. Consequently, this scarcity of evidence and assortment of opinions amongst practitioners has resulted in a wide variance in practice patterns and general discomfort surrounding PN administration and timing of initiation. Two randomized controlled trials, the EPaNIC trial and the Early PN Trial, evaluated the timing of PN administration in critically ill patients admitted to the ICU, with adults >18 years of age eligible for inclusion (28, 29). Neither study found a reduction in mortality or infection rates with early PN. In the EPaNIC trial, late initiation of PN was associated with several benefits, including shorter duration of mechanical ventilation, shorter duration of renal replacement therapy, shorter ICU stay, shorter hospital stay, and reduced health care costs (28). Importantly, this trial enrolled critically ill adults to begin PN regardless of the adequacy of enteral intake, with the dose of PN targeted to meet 100% of a patient’s caloric goal through combined PN and EN. In contrast, our study primarily focused on patients with contraindications or intolerance to EN, with only 3 of the 58 patients receiving EN simultaneously with PN. The Early PN Trial also focused on this subset of patients unlikely to receive any early EN. Both medical and surgical patients were included, with 65% of patients requiring surgery. Results demonstrated that early administration of PN was protective against both muscle wasting and fat loss, although this did not translate into improvements in physical function or mortality at 60 days (29).
EGS patients frequently present with complex intraabdominal processes that prevent the immediate use of the gastrointestinal tract. Therefore, PN is often the only option for many EGS patients presenting with intestinal obstruction, perforation, or other pathology that prohibits adequate oral or enteral intake (30). Historically, concerns regarding PN-associated complications were commonly cited reasons for delaying PN initiation or avoiding its use altogether. However, much of the data outlining these risks are derived from dated studies conducted in the 1980s to early 2000s (21). Studies performed within the most recent decade have failed to demonstrate the same rates of infection, potentially due to improved glycemic control as well as improved sterile technique and central line care (18, 20). Likewise, increasing access to inpatient nutrition consult services and better education amongst healthcare providers have assisted in proper management and avoidance of PN-related metabolic disturbances and nutritional derangements. Furthermore, some of the more feared risks, such as metabolic bone disease, liver failure, and central venous access complications, are more frequently associated with long-term, chronic use of PN and less likely to occur in inpatients requiring short-term therapy (31). In the present study, complications related to PN were negligible following a median duration of use of 10 days. Additionally, no patients in this cohort demonstrated metabolic or electrolyte derangements that necessitated premature cessation of PN therapy. Therefore, in the setting of short-term, in-hospital use, PN-associated complications may be less of a concern than initially suggested.
In addition to demonstrating a nominal rate of PN-related complications, this study also revealed a potential benefit of early PN use in this particular population. Morbidity and mortality outcomes for patients requiring emergent general surgery are an estimated 7-fold higher compared to elective surgery (32). These rates are further amplified in the elderly population, with mortality increasing every decade beyond age 50 years, reaching nearly 40-50% in patients 80 years and older (9). Our cohort demonstrated a similar trend, with an overall mortality rate of 45% for the entire group. Additionally, similar to our findings, a study by Joseph et al evaluating geriatric EGS patients demonstrated that, on average, nearly half of a patient’s hospital stay was spent in the ICU, indicating a high degree of acuity and critical care needs among this population (33). Importantly, our results revealed an impressive decrease in ICU admissions by 23.9% as well as a reduction in mortality by 21.5% in patients receiving early PN. A larger sample size will be necessary to assess whether this mortality difference is in fact significant. Additionally, patients in the early administration group were 3.2 times more likely to reach 60% of their target caloric goal within the first 72 hours of admission, indicating a positive trajectory to meeting their overall nutritional needs earlier in their hospital stay. In a study by Kim et al, critically ill surgical patients who approached their target calorie intake demonstrated significantly improved clinical outcomes (34). Harmandar et al confirmed similar results, with a lower mortality rate and improved nutritional status in those who achieved their target caloric goal compared to non-achievers (35). Therefore, given the potential for improved outcomes with earlier initiation of PN in this inherently high-risk operative group, future research in this area is a necessity.
There are several limitations to this study. First, the small case number and single-institution design limit the generalizability of our results. Additionally, the findings must be interpreted with caution due to the methodological limitations associated with the retrospective, observational design. While the cohorts were well matched, thus suggesting minimal selection bias, we recognize that it is impossible to eliminate residual confounding without prospective randomization. In regard to the use of the Frailty Index, while we based the development of this index on several validated studies in other ageing populations, there is little evidence evaluating the Frailty Index specifically in the EGS population. For the purposes of this study, the Frailty Index was intended to provide an objective measure of health and functional status among an elderly study cohort. Therefore, while we acknowledge the limitations of its use and need for further validation in the acute care setting, we utilized the index as one potential helpful method to globally compare and risk stratify patients across the two study groups. Additionally, it is important to note that despite an increased rate of ICU admissions among the late PN group, we observed no difference in specific postoperative outcomes, likely secondary to the small sample size, a wide assortment of complications necessitating ICU admission, or complications that were not captured by our defined outcome variables. Ultimately, a larger sample size is needed to distinguish differences in these individual outcomes. We also recognize that information regarding functional status post-hospitalization is an essential adjunct for assessing long-term morbidity. However, this was difficult to consistently ascertain from the medical chart and, therefore, was not included as an outcome variable. Although underpowered due to the small sample size, this initial study suggests that the timing of nutritional support in elderly surgical patients is an important area for future research and provides critical groundwork to inform and power future prospective analyses. Ultimately, large-scale, randomized studies are needed to validate the theoretical advantages of early PN in elderly surgical patients to expedite healing and potentially improve postoperative outcomes.
Elderly patients undergoing emergent general surgery are at high risk for malnourishment and functional decline, resulting in substantial morbidity and mortality rates (9, 32). Current guidelines fail to address PN use specifically in this high-risk age group, resulting in potentially avoidable delays in proper nutritional support in elderly surgical patients unable to receive EN. We have demonstrated that early initiation of PN in hospitalized elderly EGS patients is safe and not associated with increased adverse events compared to patients receiving delayed PN. Furthermore, our findings demonstrate a significant decrease in critical care utilization and a trend toward improved mortality among patients receiving early PN. Based on these findings, and with the lack of existing recommendations guiding the use of PN in this age group, larger prospective studies are warranted to further explore the safety and potential benefits of early PN administration in elderly surgical patients unable to receive EN.
Disclosures: None.
Sources of Funding: This research was supported by K08GM134185 (JPI) and R01AG018859 (EJK). The content in this report is the responsibility of the authors and does not represent the formal National Institutes of Health opinion.
Conflict of Interest: There are no conflicts of interest to report.
Ethical standards: All procedures performed involving human participants were in accordance with the ethical standards of the Colorado Multiple Institutional Review Board and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
1. Abunnaja S, Cuviello A, Sanchez JA. Enteral and parenteral nutrition in the perioperative period: state of the art. Nutrients. 2013;5(2):608-23, doi: 10.3390/nu5020608.
2. McClave SA, Taylor BE, Martindale RG, et al. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient. J Parenter Enteral Nutr. 2016;40(2):159-211, doi: 10.1177/0148607115621863.
3. Meadows N. Monitoring and complications of parenteral nutrition. Nutrition. 1998;14(10):806-8, doi: 10.1016/s0899-9007(98)00089-6.
4. Wu AW, Yasui Y, Alzola C, et al. Predicting functional status outcomes in hospitalized patients aged 80 years and older. J Am Geriatr Soc. 2000;48(S1):S6-15, doi: 10.1111/j.1532-5415.2000.tb03142.x.
5. Freeman W, Weiss AJ, Heslin KC. Overview of U.S. Hospital Stays in 2016: Variation by Geographic Region. HCUP Statistical Brief #246. Agency for Healthcare Research and Quality, Rockville, MD, Dec 2018.
6. He W, Goodkind, D, Kowal, P. An Aging World: 2015. US Census Bureau, International Population Reports, P95/16-2. U.S. Government Publishing Office, Washington, DC, 2016.
7. Beadles CA, Meagher AD, Charles AG. Trends in Emergent Hernia Repair in the United States. JAMA Surg. 2015;150(3):194-200, doi: 10.1001/jamasurg.2014.1242.
8. Thorsen K, Søreide JA, Kvaløy JT, Glomsaker T, Søreide K. Epidemiology of perforated peptic ulcer: age- and gender-adjusted analysis of incidence and mortality. World J Gastroenterol. 2013;19(3):347-54, doi: 10.3748/wjg.v19.i3.347.
9. Desserud KF, Veen T, Søreide K. Emergency general surgery in the geriatric patient. Br J Surg. 2015;103(2):e52-e61,doi: 10.1002/bjs.10044.
10. Avelino-Silva TJ, Farfel JM, Curiati JAE, Amaral JRG, Campora F, Jacob-Filho W. Comprehensive geriatric assessment predicts mortality and adverse outcomes in hospitalized older adults. BMC Geriatr. 2014;14(1):129, doi: 10.1186/1471-2318-14-129.
11. Paillaud E, Herbaud S, Caillet P, Lejonc JL, Campillo B, Bories PN. Relations between undernutrition and nosocomial infections in elderly patients. Age Ageing. 2005;34(6):619-25, doi: 10.1093/ageing/afi197.
12. Rasheed S, Woods RT. Malnutrition and Associated Clinical Outcomes in Hospitalized Patients Aged 60 and Older: An Observational Study in Rural Wales. J Nutr Gerontol Geriatr. 2013;32(1):71-80, doi: 10.1080/21551197.2012.753772.
13. Sungurtekin H, Sungurtekin U, Balci C, Zencir M, Erdem E. The influence of nutritional status on complications after major intraabdominal surgery. J Am Coll Nutr. 2004;23(3):227-32, doi: 10.1080/07315724.2004.10719365.
14. Avelino-Silva TJ, Jaluul O. Malnutrition in Hospitalized Older Patients: Management Strategies to Improve Patient Care and Clinical Outcomes. Int J Gerontol. 2017;11(2):56-61, doi.org/10.1016/j.ijge.2016.11.002.
15. Feldblum I, German L, Castel H, et al. Characteristics of undernourished older medical patients and the identification of predictors for undernutrition status. Nutr J. 2007;6:37, doi: 10.1186/1475-2891-6-37.
16. Schindler K, Pernicka E, Laviano A, et al. How nutritional risk is assessed and managed in European hospitals: A survey of 21,007 patients findings from the 2007–2008 cross-sectional nutritionDay survey. Clin Nutr. 2010;29(5):552-, doi: 10.1016/j.clnu.2010.04.001.
17. Osooli F, Abbas S, Farsaei S, Adibi P. Identifying Critically Ill Patients at Risk of Malnutrition and Underfeeding: A Prospective Study at an Academic Hospital. Adv Pharm Bull. 2019;9(2):314-20, doi: 10.15171/apb.2019.037.
18. Cotogni P, Pittiruti M, Barbero C, Monge T, Palmo A, Boggio Bertinet D. Catheter-Related Complications in Cancer Patients on Home Parenteral Nutrition. J Parenter Enteral Nutr. 2013;37(3):375-83, doi: 10.1177/0148607112460552.
19. Gandullia P, Lugani F, Costabello L, et al. Long-term home parenteral nutrition in children with chronic intestinal failure: A 15-year experience at a single Italian centre. Dig Liver Dis. 2011;43(1):28-33, doi: 10.1016/j.dld.2010.04.012.
20. Martincich I, Cini K, Lapkin S, Lord H, Fernandez R. Central Venous Access Device Complications in Patients Receiving Parenteral Nutrition in General Ward Settings: A Retrospective Analysis. J Parenter Enteral Nutr. 2020;44(6):1104-11, doi: 10.1002/jpen.1743.
21. McCleary EJ, Tajchman S. Parenteral Nutrition and Infection Risk in the Intensive Care Unit: A Practical Guide for the Bedside Clinician. Nutr Clin Pract. 2016;31(4):476-89, doi: 10.1177/0884533616653808.
22. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-83, doi: 10.1016/0021-9681(87)90171-8.
23. Walston J, Hadley EC, Ferrucci L, et al. Research Agenda for Frailty in Older Adults: Toward a Better Understanding of Physiology and Etiology: Summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc. 2006;54(6):991-1001, doi: 10.1111/j.1532-5415.2006.00745.x.
24. Xue Q-L. The frailty syndrome: definition and natural history. Clin Geriatr Med. 2011;27(1):1-15, doi: 10.1016/j.cger.2010.08.009.
25. Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci. 2007;62(7):722-7, doi: 10.1093/gerona/62.7.722.
26. Song X, Mitnitski A, Rockwood K. Prevalence and 10-year outcomes of frailty in older adults in relation to deficit accumulation. J Am Geriatr Soc. 2010;58(4):681-7, doi: 10.1111/j.1532-5415.2010.02764.x.
27. Cheung A, Haas B, Ringer TJ, McFarlan A, Wong CL. Canadian Study of Health and Aging Clinical Frailty Scale: Does It Predict Adverse Outcomes among Geriatric Trauma Patients? J Am Coll Surg. 2017;225(5):658-65.e3, doi: 10.1016/j.jamcollsurg.2017.08.008.
28. Casaer MP, Mesotten D, Hermans G, et al. Early versus Late Parenteral Nutrition in Critically Ill Adults. N Engl J Med. 2011;365(6):506-17, doi: 10.1056/NEJMoa1102662.
29. Doig GS, Simpson F, Sweetman EA, et al. Early Parenteral Nutrition in Critically Ill Patients With Short-term Relative Contraindications to Early Enteral Nutrition: A Randomized Controlled Trial. JAMA. 2013;309(20):2130-8, doi: 10.1001/jama.2013.5124.
30. Ashmore D, Lee M. Parental nutrition in emergency surgery: a multicentre cross-sectional study. J Hum Nutr Diet. 2021, doi: 10.1111/jhn.12902.
31. Dibb M, Teubner A, Theis V, Shaffer J, Lal S. Review article: the management of long-term parenteral nutrition. Aliment Pharmacol Ther. 2013;37(6):587-603, doi: 10.1111/apt.12209.
32. Shah AA, Haider AH, Zogg CK, et al. National estimates of predictors of outcomes for emergency general surgery. J Trauma Acute Care Surg. 2015;78(3), doi: 10.1097/TA.0000000000000555.
33. Joseph B, Zangbar B, Pandit V, et al. Emergency General Surgery in the Elderly: Too Old or Too Frail? J Am Coll Surg. 2016;222(5):805-13, doi: 10.1016/j.jamcollsurg.2016.01.063.
34. Kim MK, Choi YS, Suh SW, Lee SE, Park YG, Kang H. Target Calorie Intake Achievements for Patients Treated in the Surgical Intensive Care Unit. Clin Nutr Res. 2021;10(2):107-14, doi: 10.7762/cnr.2021.10.2.107.
35. Harmandar FA, Gömceli I, Yolcular BO, Çekin AH. Importance of target calorie intake in hospitalized patients. Turk J Gastroenterol. 2017;28(4):289-97, doi: 10.5152/tjg.2017.16718.