A. Jullien1, A. Pagès1,2,3,*, C. Cantet2, G. Soriano2,3, L. Rouch1,2,3, C. Cool1,2, S. Andrieu2, N. Tavassoli3, P. Cestac1,2, Y. Rolland2,3, C. McCambridge1
1. Department of Pharmacy, Toulouse University Hospital, Toulouse, France; 2. Center for Epidemiology and Research in POPulation health (CERPOP), UMR 1295, Inserm, UPS Toulouse III University, Toulouse, France; 3. Institute of Aging, Gérontopôle, INSPIRE project, Toulouse University Hospital, Toulouse, France
Corresponding author: Arnaud Pagès, Department of Pharmacy, Toulouse University Hospital, Toulouse, France, pages.ar@chu-toulouse.fr; Tel: +33-567-776-418
Jour Nursing Home Res 2023;9:1-8
Published online April 6, 2023, http://dx.doi.org/10.14283/jnhrs.2023.1
Abstract
Background: Drugs with anticholinergic properties have many adverse effects that can affect nutritional status. Methods: An observational cross-sectional study was conducted on data from the IDEM study (N=585) to explore the link between nutritional status and anticholinergic burden as well as other factors among older nursing home residents. Nutritional status was determined using the Mini Nutritional Assessment. Multivariate logistic mixed regression was used. Results: Half of residents (N=293) had an impaired nutritional status. After adjusting for potential confounders, there was no significant association between high anticholinergic burden and impaired nutritional status. However, the impaired nutritional status was significantly higher for women (OR=1.90, 95CI=[1.15–3.15]), patients with a high Charlson Comorbidity Index score (OR=2.22, 95CI=[1.12–4.38]), history of fracture (OR=1.62, 95CI = [1.03–2.54]) or abnormal one-leg standing test (OR=1.99, 95CI=[1.04–3.78]), a low cognitive score (OR=0.95, 95CI=[0.91–1.00]), with a low autonomy scale (GIR) (OR=0.69, 95CI=[0.57–0.85]) and with a poor Quality of Life-Alzheimer’s Disease assessment (OR=0.90, 95CI=[0.86–0.94]). Conclusions: We found no significant association between anticholinergic exposure and nutritional status in older residents living in nursing homes. The use of existing scales in clinical practice remains a challenge because of the complexity of calculating anticholinergic exposure.
Key words: Mini Nutritional Assessment, anticholinergic burden, nutritional status, nursing homes, geriatrics, iatrogenic.
Introduction
The pharmaceutical treatment of nursing home residents is a public health issue. Malnutrition is prevalent in nursing home with multifactorial risk factors (1). Polypharmacy is associated to the nutritional risk (2). Drugs with anticholinergic properties are among those commonly implicated in iatrogenic illness because of their adverse effects (3). Aging physiologically alters the pharmacodynamics and pharmacokinetic properties of drugs, making older people more sensitive to adverse effects (4) including those of anticholinergics. Each anticholinergic drug can be characterized by its “anti-cholinergic burden.” The anticholinergic burden can be calculated using different rating scales. The scales have been developed using in vitro methods (serum anticholinergic activity (SAA)) and/or on the basis of empirical evidence of adverse reactions to determine the anticholinergic properties of drugs and/or on expert opinion (clinicians, pharmacists, and pharmacologists) (5). The prevalence of anticholinergic prescriptions is high (24%) in nursing home patients (6). Exposure of older subjects to anticholinergics has been associated with cognitive and functional decline, excess mortality, and recurrent falls (7). We hypothesized that adverse anticholinergic-related effects such as loss of appetite, altered sense of taste, dry mouth, dyspepsia, nausea, vomiting, constipation, or fluid electrolyte imbalances could change the nutritional status of nursing home residents (8-10). Other conditions like as dependency in activities of daily living, depression, dementia, and iatrogenic illness are particularly important risk factors of malnutrition in the older adults, and require regular evaluation of nutritional status, and more specifically, in nursing homes (11). According to a meta-analysis by Cereda et al. (12) in 2016 the nutritional status of nursing home residents is concerning: the prevalence of malnutrition was 17.5%, and the risk of malnutrition was 48%.
The association between anticholinergic burden and nutritional status in older patients is controversial with differing results across studies and countries (13, 14). The aim of this study was to evaluate whether a high anticholinergic burden was associated to an impaired nutritional status in French nursing home residents.
Materials and Methods
Design and population
We used the data from “intervention” group at baseline of the IDEM (Impact of Systematic Tracking of Dementia Cases on the Rate of Hospitalization in Emergency Care Units) study to perform a secondary analysis (15). IDEM is a French multicenter study cluster randomized by nursing home (1:1) that compared 2 groups: an intervention group consisting of nursing homes that set up multidisciplinary team meetings to identify residents with dementia and to propose an appropriate care plan, and a control group of nursing homes that continued their usual practice (ClinicalTrials.gov Identifier: NCT01569997). The study protocol was approved by the French Ethics Committee for the Protection of Persons (CPP SOOM II) located in Toulouse. Oral informed consent for study participation was obtained from all residents or their representatives. Participants’ written informed consent was not required by French law at the time of the study. This cross-sectional and observational study is reported in compliance with the STROBE guidelines (16).
All the residents of participating nursing homes who met the study criteria were included in the study: residents aged 60 years or older, without diagnosed or documented dementia, not bedridden, living in the nursing home for at least 1 month at inclusion, with a life expectancy of more than 1 year, and without any disease likely to jeopardize his or her participation in the study (15).
Sociodemographic and medical data, including pharmacological and non-pharmacological treatments, and level of dependence [Groupe Iso-Ressources (GIR) questionnaire a French level-of-dependence score from 1 to 6, where 1 indicates completely dependent or bedridden and 6 indicates completely independent] were collected by the coordinating physician in both groups during the inclusion visit. The residents in the intervention group also underwent a comprehensive geriatric assessment: cognitive assessment [Mini-Mental State Examination (MMSE), clock-drawing test, Borson’s Mini-Cog, 5-word test, test of categorical verbal fluency]; functional and physical assessment [Instrumental Activities of Daily Living (IADL), one-leg standing balance test]; nutritional assessment [Mini-Nutritional Assessment (MNA) (17)]; psychological assessment [Confusion Assessment Method (CAM), Neuropsychiatric Inventory caregiver version (NPI), Mini Geriatric Depression Scale (mini-GDS), Quality of Life–Alzheimer’s Disease (QOL-AD)] (15). We chose to analyze only the data from the «intervention» group at inclusion because the MNA, the Mini GDS and the one-leg standing balance test were only collected at that time and only in that group for the study. The medication data came from the patients’ prescriptions or from the nursing home software, we had at least the International Nonproprietary Names (INN) the trade name and the dosage form of each medication.
Outcome measures
The outcome was the MNA as evidence of the nutritional status. Nutritional status was categorized into three categories: normal nutritional status (MNA ≥ 24 points), risk of malnutrition (17 < MNA ≤ 23.5 points) or malnutrition status (MNA ≤ 17 points). Because the number of people with malnutrition status (MNA<17) was low, we chose to gather the group “malnutrition” and the group “risk of malnutrition” in a new group called “impaired nutritional status”.
For each resident, the explanatory variable was the overall anticholinergic burden at inclusion according to the Anticholinergic Drug Scale (ADS) (18). ADS scale was chosen because it is more comprehensive, less restrictive than other scales and allows calculating a score. For each resident, the overall anticholinergic burden of drug prescribing was calculated by summing the anticholinergic scores of each drug. The scores obtained were then studied in the form of a three-modality category variable with 0 for no anticholinergic activity group, 1 to 2 for moderate anticholinergic activity group and 3 for high anti-cholinergic activity group (19).
Covariables
The adjustment variables available in the IDEM cohort were:
– Sociodemographic characteristics with age and sex
– Medical history with comorbidities evaluated using the Charlson Comorbidity Index score (20); personal psychiatric history, depression, bipolar disorder, psychosis, acute confusional episode, and other psychiatric history; History of fracture and osteoporosis with a high risk for fractures, family history of dementia
– Standardized gerontological assessments with cognitive assessment (MMSE) and noncognitive assessment with the mini-GDS, the NPI, the GIR questionnaire, the one-leg standing balance test, and the QOL-AD scale, from which two items, “relationship with spouse” and “household maintenance” were excluded because they did not apply to nursing home residents according to the method of Logsdon et al. (21). In addition, for the QOL-AD scale, as proposed by Logdson et al. (21) missing items were replaced by the mean score of the remaining items if 1 or 2 items were missing; otherwise the score was considered missing.
– Nursing home characteristics such as the status of the establishment (Public, Private, Private but nonprofit), presence of a special care unit, and the time the resident has been in the nursing home.
Statistical analysis
The number of missing data for the variable to be explained (MNA) was very low (N=14). Assuming a completely random mechanism for the missing data, we chose not to impute these data. Therefore, 585 residents were included in the analysis. To describe the data, we provided the mean ± standard deviation for quantitative variables and counts (%) for qualitative variables.
We performed a bivariate analysis to look for all the factors associated with nutritional status (MNA in 2 classes: ≤23.5 versus >23.5). For this analysis, we used the Chi-square test to examine the association between nutritional status and the qualitative variables. For the quantitative variables, we used the Student test for Gaussian distributions and the Mann-Whitney nonparametric test for non-Gaussian distributions.
To determine whether the anticholinergic burden was independently associated with the MNA, we conducted a multivariate analysis using a mixed logistic regression model with a random intercept on the cluster (nursing home) to consider the intra-cluster correlation. In this model, the MNA was the dependent variable in 2 classes (≤ 23.5 versus > 23.5) The ADS score was the explanatory variable of interest in 3 classes (0, 1-2, ≥ 3). The significant variables in the bivariate analysis with a threshold of 25% were included in this model in order to take into account all potential confounding factors. The significant variables in the final model were retained with a threshold of 5% using the backward stepwise procedure. The ADS score was forced into the multivariate model, as it was the variable of interest. The interactions between the ADS score and all the variables of the final model were tested and none were significant. For each variable in the final model, the odds ratio (OR) with its 95% confidence interval (CI), as well as the associated “p value” are given. To assess collinearity, tolerance from a linear regression was calculated for the final model. In a sensitivity analysis, another model was performed with variable of interest (ADS score) and variables already associated with malnutrition in the literature. The statistical analyses were performed using SAS 9.4 software (SAS Institute, Cary, North Carolina, USA).
Results
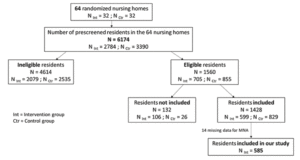
Sixty-four nursing homes participated in the IDEM study and enrolled 1428 residents: 599 in the intervention group (32 nursing homes) and 829 in the control group (32 nursing homes). (Figure 1). Population characteristics are described in Table 1.
Source: Rolland Y. – SFGG (Société Française de Gériatrie et Gérontologie) Congres, November 2016)
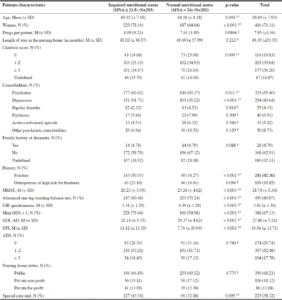
ADS scores of residents are described in Table 2. Of the residents exposed to at least one anticholinergic drug (N = 411), including 307 with an ADS score = 1-2 and 104 with an ADS score ≥3. The mean anticholinergic burden score was 1.97 ±1.36. The anticholinergic agents are listed in Table S1 (Supplementary Material). The number of residents according to their Mini Nutritional Assessment scores are presented in Figure S1 (Supplementary Material).
Abbreviation: ADS: Anticholinergic Drug Scale; Explanations: The overall anticholinergic burden of the drug prescription (ADS score) was calculated by summing the anticholinergic scores of each drug.
The results of the bivariate analysis are presented in Table 3. During the backward stepwise procedure, the variables have been removed from the model in the following order: 1) Family history of dementia, 2) Age, 3) Special care unit, 4) NPI, 5) Length of stay in the nursing home, 6) Charlson score. The results of the multivariate analysis are shown in Table 4 and suggest there is no statistically significant association between anticholinergic burden and nutritional status. On the other hand, the impaired nutritional status is significantly higher among women, residents with a high Charlson score, a history of fracture, abnormal one-leg standing test, or a low score on the MMSE, or with a low GIR or QOL-AD assessments. For over adjustment assessment, the tolerance for each variable is presented in Table S2 (Supplementary Material). Even if there is no formal threshold for deciding if a tolerance is low enough to affect the predicted values, these values, all >0.40, suggest that there is no collinearity. Finally, the results of the sensitivity analysis model are presented in Table S3 (Supplementary Material). They are similar to the results of the main analysis.
Legend: *: significant at 5% threshold; 1: Chi-2; 2: Student’s t test; 3: Kruskal-Wallis test; Abbreviations: M: Mean; SD: standard deviation; N: Number of cases; MMSE: Mini Mental State Examination; GIR (Groupe Iso-Ressources); GDS: Geriatric Depression scale; QOL-AD: Quality of Life in Alzheimer’s Disease; NPI: NeuroPsychiatric Inventory; MNA: Mini Nutritional Assessment; ADS: Anticholinergic Drug Scale
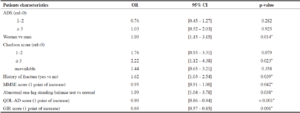
Table 4
Multivariate analysis – Association between anticholinergic burden and impaired nutritional status (MNA≤23.5, N=235) –
Final model (N=499)
Additional information: Coefficient of Determination R2=0.2887 (formula from Nagelkerke, N. J. D. (1991). A Note on a General Definition of the Coefficient of Determination. Biometrika 78:691–692.); *: significant at 5% threshold; Abbreviations: ADS: anticholinergic drug scale; OR: Odds Ratio; CI: Confidence Interval; MMSE: Mini Mental State Examination; QOL-AD: Quality of Life in Alzheimer’s Disease; GIR (Groupe Iso-Ressources)
Discussion
Our study found no association between anticholinergic burden and nutritional status. The association between anticholinergic burden and nutritional status among older patients is controversial with differing results across studies and countries. For example, in the Aalto et al. study performed in Finland (14), the authors showed no association between anticholinergic burden assessed with the Anticholinergic Risk Scale (ARS) (19) and nutritional status assessed with the MNA (17). In contrast, in the work of Kose et al. carried out in Japan (13), the authors found an association between increased anticholinergic burden assessed with the ARS scale (19), and poor nutritional status assessed with the Geriatric Nutritional Risk Index (GNRI) (22). Thus, these differences in outcomes could be related to differences in tools for assessing nutritional status and anticholinergic burden, but also to differences between the older populations under consideration and between countries. It is difficult to transpose these results to our study because the two scales ADS and ARS differ as to their sensitivity and their specificity (23). It is also difficult to compare these results with our study on nutritional status because GNRI and MNA are different (24).
Moreover, we observed a significant increase in the impaired nutritional status in dependent subjects. This result is consistent with the work of Lang et al. (25). However, autonomy was not assessed by the GIR questionnaire but by the Katz’s ADL (Activities of Daily Living) index and the population were not nursing home residents but community dwelling seniors. Another study showed similar results with the Barthel Index (26). However, the Katz’s ADL as well as the Barthel Index do not take into account the loss of cognitive autonomy unlike the GIR questionnaire. Then, the loss of cognitive autonomy can have an impact on the nutritional status (27). In our study, residents with cognitive impairment or a large number of comorbidities had a significantly higher rate of impaired nutritional status, like in the Peng et al. study (28). In addition, the dementia was diagnosed for 574 residents in the IDEM study (97.6%): high probability of dementia for 129 residents (22.5%) and suspicion of dementia for 107 residents (18.6%) (15). The relationship between high levels of anticholinergic burden and the occurrence of dementia is not always demonstrated both with the same scales as our study (ADS and MMSE) (29, 30) and others (Anticholinergic Cognitive Burden Scale (ACB), Drug Burden Index (DBI) and Clinical Practice Research Datalink (CPRD)) (31, 32). Differences in the diagnosis of cognitive disorders and data on medications (doses, duration of intake, etc.) could limit the comparability of results between studies. Indeed, it is also important to highlight that the anticholinergic burden may worsen pre-existing cognitive disorders (33). We also found a significantly higher impaired nutritional status in residents with an abnormal one-leg standing test. The work of Neyens et al. (34) showed that patients who fall had a significantly higher risk of malnutrition. However, very few studies have shown an association between fall risk and nutritional status. In residents with a history of fracture, we observed a significantly higher risk of being malnourished compared with residents who did not have a history of fracture. These results are similar to those of the Mastronuzzi et al. study (35). The anticholinergic exposure could be also connected with an increased mortality risk (36). Our work showed residents with a low QOL-AD score have a significantly higher risk. Another study reported similar results, however, it is difficult to compare our results to this study because they were not the same population (patients with non-small cell bronchial cancer), nor the same scale (Quality of Life Questionnaire scale-Lung Cancer 13 or QLQ-LC 13 specific to oncology). In our study, several limitations need to be taken into account. First, it is a cross-sectional and observational study in which the length of exposure to anticholinergics was not evaluated. Other studies were also cross-sectional or longitudinal but with a short follow-up period (less than 3 months). Longitudinal studies with an extended follow-up period will be needed in the future to determine whether long-term exposure to anticholinergic drugs leads to a deterioration in nutritional status.
Nonetheless, some strengths of our study should be emphasized. It is the first study to address the association between anticholinergic burden and nutritional status in French nursing home residents. Finally, there are very few missing data and the analyses were adjusted to take the main confounding factors linked to nutritional status into account.
Conclusions
Our study evaluated the anticholinergic burden in a cohort of older people living in nursing home and found no significant association between a high anticholinergic burden and the risk of being malnourished, while taking several confounding factors into account. Large-scale longitudinal studies should be considered to monitor the exposure to anticholinergics and compare the anticholinergic burden with several existing scales. The use of existing scales in clinical practice remains a challenge because of the complexity of calculating anticholinergic exposure, the updating of scales, and the differences observed between anticholinergic burden and clinically proven adverse events.
Author Contributions: Conceptualization, A.J., C.Co., Y.R. and C.M.; methodology, A.J., A.P. and L.R.; validation, all authors; statistical analysis, C.Ca.; data curation, A.J.; writing—original draft preparation, A.J. and A.P.; writing—review and editing, all authors; supervision, S.A., P.C., Y.R. and C.M.; project administration, N.T.; funding acquisition, N.T. and Y.R. All authors have read and agreed to the published version of the manuscript.
Funding: The IDEM (Impact of Systematic Tracking of Dementia Cases on the Rate of Hospitalization in Emergency Care Units) study was supported by grants from the French Ministry of Health (PHRC 2009, No. 0910701). The promotion of this study was supported by the University Hospital Centre of Toulouse.
Institutional Review Board Statement: The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the French Ethics Committee for the Protection of Persons (CPP SOOM II) located in Toulouse (protocol code: 0910701 and registration number: 2-10-01).
Informed Consent Statement: Oral informed consent for study participation was obtained from all residents or their representatives. Participants’ written informed consent was not required by French law at the time of the study.
Data Availability Statement: No data available.
Acknowledgments: The authors thank the members of the IDEM study group (listed at the end of the manuscript).
Conflicts of Interest: The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
IDEM study group refers to: Collection, management, analysis, and interpretation of the data: Gabor Abellan Van Kan, Estelle Aliphat, Sandrine Andrieu, Fabienne Boubals, Christelle Cantet, Marie-Christine Cazes, Philipe de Souto Barreto, Maryline Duboué, Virginie Gardette, Stéphane Gérard, Sophie Guyonnet, Sophie Hermabessière, Clarisse Laffon de Mazières, Christophe Morin, Amélie Perrin, Yves Rolland, Nathalie Roques, Sandrine Sourdet, Neda Tavassoli, Elodie Tournay, Bruno Vellas, Hélène Villars, Thierry Voisin. Coordinating physicians, investigators realizing the clinical assessment and data collection in Nursing Homes: Françoise Armengaud, Véronique Arnaud-Ulliet, Jean-Paul Bacque, Rodica Balan, Nadia Bannour, Marie-France Basset-Berges, Didier Benhamou, Serge Bismuth, Dominique Blanc, René Bonnet, Christine Chansiaux, Jacques Courivaud, Jean-Christian Boubenne, Violaine Bousquet, Bernard Bryckaert, Gérard Bury, Fabienne Calvière-Lano, Luc Cantaloube, Josiane Charrière, Carine Chiffre, Alain Chiron, Pierre Codron, Jacques Coll, Magalie Coppens, Martine Couderc, Dominique Dantoine, Candida Delmas, Louis Déroudille, Michel Drugeon, Jean-Paul Duffau, Christian Fabié, Jean Favarel, Benoit Gavoille, Hervé Gay, Jean-François Gaye-Palettes, Mustafa Gokpinar, Aurelia Gonnot, Françoise Guillemette, Wassim Hamie, Sophie Hervochon-Cosson, Michel Idrac, Jean-François Jammes, Adrian Klapouszczak, Denis Lagarde, Bernard Lagarrigue, Hafida Lallem, Elise Laspreses, Jean-Marie Laur, Beatrice Laurent, Jean-Marc Laurent, Paule Le Deun, Frédéric Leclercq, Mickael Li Yung Tong, Jean-Luc Louis, Pierre Marty-Faucher, Martine Merceron, Juliette Meunier, Thierry Montemayor, Jean-Pierre Moulinié, Caroline Parneix, Jean-Claude Passebon, Alain Peborde, Michèle Péré-Saun, Isabelle Perus, Jean-Luc Philip, Mathieu Pinar, François Pinoche, Corinne Quintana, Christophe Schreyer, Albert Siboni, Elisabeth Sonneck, Martine Soudani Le Noc, Kristel Sudres-Scheirlinckx, Georges Teisseyre, Patrice Teste, Valérie Vayrac, Pierre Viguier, Sylvie Vrignaud. Expert physicians of the memory clinics participating in Multidisciplinary Team Meetings: Gabor Abellan Van Kan, Christophe Arbus, Jean-François Audusseau, Karim Bennys, Lawrence Bories, Jean-Pierre Calmels, Martine Camallières, Marie-Noëlle Cufi, Julien Delrieu, Stéphane Gérard, Christophe Hein, Sophie Hermabessière, Mathieu Houles, Claude Jeandel, Clarisse Laffon de Mazières, Jean-Paul Lembelembe, Philippe Martinez, Fati Nourhashemi, Pierre-Jean Ousset, Caroline Parneix, Amélie Perrin, Alain Quinçon, Philippe Robert, Yves Rolland, Serge Sainte-Foie, Nathalie Sastre, Sandrine Sourdet, Jacques Touchon, Marc Verny, Hélène Villars, Luc Vogt, Thierry Voisin, Lisette Volpe-Gillot.
Open Access: This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
References
1. Bell, C.L.; Lee, A.S.W.; Tamura, B.K. Malnutrition in the Nursing Home. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 17–23, doi:10.1097/MCO.0000000000000130.
2. Zadak, Z.; Hyspler, R.; Ticha, A.; Vlcek, J. Polypharmacy and Malnutrition. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 50–55, doi:10.1097/MCO.0b013e32835b612e.
3. Renom-Guiteras, A.; Meyer, G.; Thürmann, P.A. The EU(7)-PIM List: A List of Potentially Inappropriate Medications for Older People Consented by Experts from Seven European Countries. Eur. J. Clin. Pharmacol. 2015, 71, 861–875, doi:10.1007/s00228-015-1860-9.
4. Kompoliti, K.; Goetz, C.G. Neuropharmacology in the Elderly. Neurol. Clin. 1998, 16, 599–610.
5. Mebarki, S.; Trivalle, C. Assessment scales for the anticholinergic effects of drugs [Échelles d’évaluation de l’effet anti-cholinergique des médicaments]. NPG Neurol. – Psychiatr. – Geria. 2012, 12, 131–138, doi:10.1016/j.npg.2012.03.002.
6. Beuscart, J.-B.; Dupont, C.; Defebvre, M.-M.; Puisieux, F. Potentially Inappropriate Medications (PIMs) and Anticholinergic Levels in the Elderly: A Population Based Study in a French Region. Arch. Gerontol. Geriatr. 2014, 59, 630–635, doi:10.1016/j.archger.2014.08.006.
7. Salahudeen, M.S.; Duffull, S.B.; Nishtala, P.S. Anticholinergic Burden Quantified by Anticholinergic Risk Scales and Adverse Outcomes in Older People: A Systematic Review. BMC Geriatr. 2015, 15, doi:10.1186/s12877-015-0029-9.
8. O’Keeffe, M.; Kelly, M.; O’Herlihy, E.; O’Toole, P.W.; Kearney, P.M.; Timmons, S.; O’Shea, E.; Stanton, C.; Hickson, M.; Rolland, Y.; et al. Potentially Modifiable Determinants of Malnutrition in Older Adults: A Systematic Review. Clin. Nutr. Edinb. Scotl. 2018, doi:10.1016/j.clnu.2018.12.007.
9. Tiisanoja, A.; Syrjälä, A.-M.; Komulainen, K.; Lampela, P.; Hartikainen, S.; Taipale, H.; Knuuttila, M.; Ylöstalo, P. Anti-cholinergic Burden and Dry Mouth among Finnish, Community-Dwelling Older Adults. Gerodontology 2018, 35 (1), 3–10. https://doi.org/10.1111/ger.12304.
10. Rhodus, N. L. Nutritional Intake in Both Free-Living and Institutionalized Older Adults with Xerostomia. J. Nutr. Elder. 1991, 10 (1), 1–32. https://doi.org/10.1300/J052v10n01_01.
11. Tamura, B.K.; Bell, C.L.; Masaki, K.H.; Amella, E.J. Factors Associated with Weight Loss, Low BMI, and Malnutrition among Nursing Home Patients: A Systematic Review of the Literature. J. Am. Med. Dir. Assoc. 2013, 14, 649–655, doi:10.1016/j.jamda.2013.02.022.
12. Cereda, E.; Pedrolli, C.; Klersy, C.; Bonardi, C.; Quarleri, L.; Cappello, S.; Turri, A.; Rondanelli, M.; Caccialanza, R. Nutritional Status in Older Persons According to Healthcare Setting: A Systematic Review and Meta-Analysis of Prevalence Data Using MNA®. Clin. Nutr. 2016, 35, 1282–1290, doi:10.1016/j.clnu.2016.03.008.
13. Kose, E.; Hirai, T.; Seki, T.; Yasuno, N. Anticholinergic Load and Nutritional Status in Older Individuals. J. Nutr. Health Aging 2020, 24, 20–27, doi:10.1007/s12603-019-1283-x.
14. Aalto, U.L.; Roitto, H.-M.; Finne-Soveri, H.; Kautiainen, H.; Pitkälä, K. Use of Anticholinergic Drugs and Its Relationship With Psychological Well-Being and Mortality in Long-Term Care Facilities in Helsinki. J. Am. Med. Dir. Assoc. 2018, 19, 511–515, doi:10.1016/j.jamda.2017.11.013.
15. Rolland, Y.; Tavassoli, N.; de Souto Barreto, P.; Perrin, A.; Laffon de Mazières, C.; Rapp, T.; Hermabessière, S.; Tournay, E.; Vellas, B.; Andrieu, S. Systematic Dementia Screening by Multidisciplinary Team Meetings in Nursing Homes for Reducing Emergency Department Transfers: The IDEM Cluster Randomized Clinical Trial. JAMA Netw. Open 2020, 3, e200049, doi:10.1001/jamanetworkopen.2020.0049.
16. von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. BMJ 2007, 335, 806–808, doi:10.1136/bmj.39335.541782.AD.
17. Vellas, B.; Guigoz, Y.; Garry, P.J.; Nourhashemi, F.; Bennahum, D.; Lauque, S.; Albarede, J.L. The Mini Nutritional Assessment (MNA) and Its Use in Grading the Nutritional State of Elderly Patients. Nutr. Burbank Los Angel. Cty. Calif 1999, 15, 116–122.
18. Carnahan, R.M.; Lund, B.C.; Perry, P.J.; Pollock, B.G.; Culp, K.R. The Anticholinergic Drug Scale as a Measure of Drug-Related Anticholinergic Burden: Associations with Serum Anticholinergic Activity. J. Clin. Pharmacol. 2006, 46, 1481–1486, doi:10.1177/0091270006292126.
19. Rudolph, J.L.; Salow, M.J.; Angelini, M.C.; McGlinchey, R.E. The Anticholinergic Risk Scale and Anticholinergic Adverse Effects in Older Persons. Arch. Intern. Med. 2008, 168, 508–513.
20. Charlson, M.; Szatrowski, T.P.; Peterson, J.; Gold, J. Validation of a Combined Comorbidity Index. J. Clin. Epidemiol. 1994, 47, 1245–1251.
21. Logsdon, R.G.; Gibbons, L.E.; McCurry, S.M.; Teri, L. Assessing Quality of Life in Older Adults with Cognitive Impairment. Psychosom. Med. 2002, 64, 510–519.
22. Bouillanne, O.; Morineau, G.; Dupont, C.; Coulombel, I.; Vincent, J.-P.; Nicolis, I.; Benazeth, S.; Cynober, L.; Aussel, C. Geriatric Nutritional Risk Index: A New Index for Evaluating at-Risk Elderly Medical Patients. Am. J. Clin. Nutr. 2005, 82, 777–783, doi:10.1093/ajcn/82.4.777.
23. Gouraud-Tanguy, A.; Berlioz-Thibal, M.; Brisseau, J.-M.; Ould Aoudia, V.; Beauchet, O.; Berrut, G.; De Decker, L. [Analysis of iatrogenic risk related to anticholinergic effects using two scales in acute geriatric inpatient unit]. Geriatr. Psychol. Neuropsychiatr. Vieil. 2012, 10 (1), 27–32. https://doi.org/10.1684/pnv.2012.0337.
24. Durán Alert, P.; Milà Villarroel, R.; Formiga, F.; Virgili Casas, N.; Vilarasau Farré, C. Assessing Risk Screening Methods of Malnutrition in Geriatric Patients: Mini Nutritional Assessment (MNA) versus Geriatric Nutritional Risk Index (GNRI). Nutr. Hosp. 2012, 27 (2), 590–598. https://doi.org/10.1590/S0212-16112012000200036.
25. Lang, P.-O.; Meyer, N.; Heitz, D.; Dramé, M.; Jovenin, N.; Ankri, J.; Somme, D.; Novella, J.-L.; Gauvain, J.-B.; Couturier, P.; et al. Loss of Independence in Katz’s ADL Ability in Connection with an Acute Hospitalization: Early Clinical Markers in French Older People. Eur. J. Epidemiol. 2007, 22, 621–630, doi:10.1007/s10654-007-9150-1.
26. O’Shea, E.; Trawley, S.; Manning, E.; Barrett, A.; Browne, V.; Timmons, S. Malnutrition in Hospitalised Older Adults: A Multicentre Observational Study of Prevalence, Associations and Outcomes. J. Nutr. Health Aging 2017, 21, 830–836, doi:10.1007/s12603-016-0831-x.
27. Fanello, S.; Foucault, S.; Delbos, V.; Jousset, N. [Evaluation of nutritional status in hospitalized aged persons]. Sante Publique Vandoeuvre–Nancy Fr. 2000, 12 (1), 83–90.
28. Peng, L.-N.; Cheng, Y.; Chen, L.-K.; Tung, H.-H.; Chu, K.-H.; Liang, S.-Y. Cognition and Social-Physiological Factors Associated with Malnutrition in Hospitalized Older Adults in Taiwan. J. Nurs. Res. JNR 2015, 23, 1–5, doi:10.1097/jnr.0000000000000074.
29. Chatterjee, S.; Bali, V.; Carnahan, R.M.; Johnson, M.L.; Chen, H.; Aparasu, R.R. Anticholinergic Medication Use and Risk of Dementia Among Elderly Nursing Home Residents with Depression. Am. J. Geriatr. Psychiatry Off. J. Am. Assoc. Geriatr. Psychiatry 2016, 24, 485–495, doi:10.1016/j.jagp.2015.12.011.
30. André, L.; Gallini, A.; Montastruc, F.; Coley, N.; Montastruc, J.-L.; Vellas, B.; Andrieu, S.; Gardette, V.; MAPT/DSA study group Anticholinergic Exposure and Cognitive Decline in Older Adults: Effect of Anticholinergic Exposure Definitions in a 3-Year Analysis of the Multidomain Alzheimer Preventive Trial (MAPT) Study. Br. J. Clin. Pharmacol. 2019, 85, 71–99, doi:10.1111/bcp.13734.
31. Richardson, K.; Fox, C.; Maidment, I.; Steel, N.; Loke, Y.K.; Arthur, A.; Myint, P.K.; Grossi, C.M.; Mattishent, K.; Bennett, K.; et al. Anticholinergic Drugs and Risk of Dementia: Case-Control Study. BMJ 2018, 361, k1315, doi:10.1136/bmj.k1315.
32. Sanders, L.M.J.; Hortobágyi, T.; van Staveren, G.; Taxis, K.; Boersma, F.; Klein, H.C.; Bossers, W.J.R.; Blankevoort, C.G.; Scherder, E.J.A.; Van der Zee, E.A.; et al. Relationship between Drug Burden and Physical and Cognitive Functions in a Sample of Nursing Home Patients with Dementia. Eur. J. Clin. Pharmacol. 2017, 73, 1633–1642, doi:10.1007/s00228-017-2319-y.
33. Dyer, A. H.; Murphy, C.; Segurado, R.; Lawlor, B.; Kennelly, S. P.; NILVAD Study Group. Is Ongoing Anticholinergic Burden Associated With Greater Cognitive Decline and Dementia Severity in Mild to Moderate Alzheimer’s Disease? J. Gerontol. A. Biol. Sci. Med. Sci. 2020, 75 (5), 987–994. https://doi.org/10.1093/gerona/glz244.
34. Neyens, J.; Halfens, R.; Spreeuwenberg, M.; Meijers, J.; Luiking, Y.; Verlaan, G.; Schols, J. Malnutrition Is Associated with an Increased Risk of Falls and Impaired Activity in Elderly Patients in Dutch Residential Long-Term Care (LTC): A Cross-Sectional Study. Arch. Gerontol. Geriatr. 2013, 56, 265–269, doi:10.1016/j.archger.2012.08.005.
35. Mastronuzzi, T.; Paci, C.; Portincasa, P.; Montanaro, N.; Grattagliano, I. Assessing the Nutritional Status of Older Individuals in Family Practice: Evaluation and Implications for Management. Clin. Nutr. Edinb. Scotl. 2015, 34, 1184–1188, doi:10.1016/j.clnu.2014.12.005.
36. Chatterjee, S.; Bali, V.; Carnahan, R.M.; Chen, H.; Johnson, M.L.; Aparasu, R.R. Risk of Mortality Associated with Anti-cholinergic Use in Elderly Nursing Home Residents with Depression. Drugs Aging 2017, doi:10.1007/s40266-017-0475-5.